|
|
|
|
Introduction
Autoimmune thyroid disease (AITD) causes cellular damage and alters thyroid
gland function by humoral and cell-mediated mechanisms. Cellular damage occurs
when sensitized T-lymphocytes and/or autoantibodies bind to thyroid cell
membranes causing cell lysis and inflammatory reactions. Alterations in thyroid
gland function result from the action of stimulating or blocking autoantibodies
on cell membrane receptors. Three principal thyroid autoantigens are involved in
AITD. These are thyroperoxidase (TPO), thyroglobulin (Tg) and the TSH receptor.
Other autoantigens, such as the Sodium Iodide Symporter (NIS) have also been
described, but as yet have no diagnostic role in thyroid
autoimmunity.[248] TSH receptor autoantibodies (TRAb) are
heterogeneous and may either mimic the action of TSH and cause hyperthyroidism
as observed in Graves' disease or alternatively, antagonize the action of TSH
and cause hypothyroidism. The latter occurs most notably in the neonate as a
result of a mother with antibodies due to AITD. TPO antibodies (TPOAb) appear
involved in the tissue destructive processes associated with the hypothyroidism
observed in Hashimoto's and atrophic thyroiditis. The appearance of TPOAb
usually precedes the development of thyroid dysfunction. Some studies suggest
that TPOAb may be cytotoxic to the thyroid.[249,250] The pathologic
role of TgAb remains unclear. In iodide sufficient areas, TgAb is primarily
determined as an adjunct test to serum Tg measurement, because the presence of
TgAb can interfere with the methods that quantitate Tg [Section-3 E6]. In iodide
deficient areas, serum TgAb measurements may be useful for detecting
autoimmune thyroid disease in patients with a nodular goiter and for monitoring
iodide therapy for endemic goiter.
Laboratory tests that determine the cell-mediated aspects of the autoimmune
process are not currently available. However, tests of the humoral response,
i.e. thyroid autoantibodies, can be assessed in most clinical laboratories.
Unfortunately, the diagnostic and prognostic use of thyroid autoantibody
measurements is hampered by technical problems as discussed below. Although
autoantibody tests have inherent clinical utility in a number of clinical
situations, these tests should be selectively employed.
Clinical Significance of Thyroid Autoantibodies
TPOAb and/or TgAb are frequently present in the sera of patients with
AITD.[251] However, occasionally patients with AITD have negative
thyroid autoantibody test results. TRAb are present in most patients with a
history of or who currently have Graves' disease. During pregnancy, the presence
of TRAb is a risk factor for fetal or neonatal thyroid dysfunction as a result
of the transplacental passage of maternal TRAb.[252,253] The
prevalence of thyroid autoantibodies is increased when patients have non-thyroid
autoimmune diseases such as type 1 diabetes and pernicious
anemia.[254] Aging is also associated with the appearance of thyroid
autoantibodies and increased prevalence of AITD.[255] The clinical
significance of low levels of thyroid autoantibodies in euthyroid subjects is
still unknown.[256] However, longitudinal studies suggest that TPOAb
may be a risk factor for future thyroid dysfunction, including post-partum
thyroiditis (PPT) as well as the development of autoimmune complications from
treatment by a number of therapeutic agents.[50,257,258] These
include amiodarone therapy for heart disease, interferon-alpha therapy for
chronic hepatitis C and lithium therapy for psychiatric
disorders.[75,259-262] The use of thyroid autoantibody measurements
for monitoring the treatment for AITD is generally not
recommended.[263] This is not surprising since treatment of AITD
addresses the consequence (thyroid dysfunction) and not the cause (autoimmunity)
of the disease. However, changes in autoantibody concentrations often reflect a
change in disease activity.
Nomenclature of Thyroid Antibody Tests
The nomenclature used for thyroid autoantibodies has proliferated,
particularly in the case of TSH receptor antibodies (LATS, TSI, TBII, TSH-R and
TRAb). The terms used in this monograph, TgAb, TPOAb and TRAb are those
recommended internationally. These terms correspond to the molecular entities
(immunoglobulins) which react with the specified autoantigens recognized by the
laboratory test. Method differences may bias the measurement of these molecular
entities, e.g.: methods may detect only IgG or IgG plus IgM; TPOAb or Ab
directed to TPO and other membrane autoantigens; TSH inhibiting and/or TSH
stimulating TRAb.
Specificity of Thyroid Antibody Tests
The use of thyroid autoantibody measurements has been hampered by specificity
problems. Studies show that results vary widely depending on the method used.
This is due to differences in both the sensitivity and specificity of the
methods and the absence of adequate standardization. In the past few years,
studies at the molecular level have shown that autoantibodies react with their
target autoantigens, by binding to "conformational" domains or epitopes. The
term "conformational" refers to the requirement for a specific three-dimensional
structure for each of the epitopes recognized by the autoantibodies.
Accordingly, assay results critically depend on the molecular structure of the
antigen used in the test. Small changes in the structure of a given epitope may
result in a decrease or a loss in autoantigen recognition by the antibodies
targeted to this epitope. Recently, dual specificity TGPO antibodies, that
recognize both Tg and TPO, have been demonstrated in the blood of patients with
AITD.[264]
Guideline 29. Thyroid Antibody Method Sensitivity & Specificity
Differences
-
Recognize and understand that the results of thyroid antibody tests are
method-dependent.
-
Thyroid antibody methods recognize different epitopes in the heterogeneous
antibody populations present in serum.
-
Thyroid antibody assay differences reflect different receptor preparations
(receptor assays) or cells (bioassays) used in the assay.
-
Assay differences can result from contamination of the antigen reagent with
other autoantigens.
-
Assay differences can result from the inherent assay design (i.e. competitive
versus non-competitive immunoassay) as well as the signal used.
-
Assay differences can result from the use of different secondary
standards.
It has been known for years that autoantibodies are directed against few
epitopes as compared to heterologous antibodies. Current methods differ widely
in epitope recognition. Specificity differences can result from misrecognition
of an epitope that leads to a bias regarding the autoantibody population tested.
This results in vastly different reference intervals, even when methods are
standardized to the same international reference preparation. Whatever the
targeted autoantigen, thyroid autoantibodies are clearly not unique molecular
entities but, rather, mixtures of immunoglobulins that only have in common their
ability to interact with Tg, TPO or the TSH receptor.
Differences in the sensitivity of autoantibody tests may arise from the
design of the assay (e.g. competitive RIA versus two-site IMA) as well as the
physical method used for the signal (e.g. radioisotope versus
chemiluminiscence). Differences in specificity may occur as a result of
contamination of the autoantigen preparation by other autoantigens (e.g. thyroid
microsomes versus purified TPO). Further, misrecognition of an epitope may lead
to an underestimation of the total amount of circulating autoantibody present,
resulting in decreased sensitivity.
Guideline 30. Functional Sensitivity of Thyroid Antibody Tests
Functional sensitivity of thyroid autoantibody tests should:
-
Be determined with human serum pools containing a low autoantibody
concentration
-
Be determined using the same protocol as described for TSH (Guideline 20) but
with the between-run precision assessment made over a 6 to 12 month time-period
to represent the appropriate clinical assessment
interval.
Functional sensitivity should be determined with human serum pools containing
a low autoantibody concentration. The protocol for functional sensitivity should
be the same protocol as described for TSH (Guideline 20). The between-run
precision for TgAb tests used for monitoring TgAb-positive DTC patients should
be assessed across a longer time-period (6 to 12 months) consistent with the
interval used for serial monitoring in clinical practice.
Standardization of Thyroid Antibody Tests
Standardization of thyroid autoantibody tests is currently suboptimal.
International Reference Preparations, MRC 65/93 for TgAb, MRC 66/387 for TPOAb
are available from the National Council for Biological Standards and Control in
London, UK (www.mrc.ac.uk).
These preparations were made from a pool of serum from patients with autoimmune
thyroid disease and were prepared and lyophilized 35 years ago!
Guideline 31. For Manufacturers Standardizing Thyroid Antibody
Assays
-
Assays should be standardized against MRC International Reference
Preparations:- MRC 65/93 for TgAb, MRC 66/387 for TPOAb and MRC 90/672 for
TRAb
-
New International Reference Preparations should be prepared for TgAb and
TPOAb.
-
Secondary standards should be fully characterized to avoid bias between
different methods.
-
Reference preparations or recombinant antigen preparations should be used
when available.
It is well known that lyophilized antibodies are prone to degradation over
time. Degradation of the antibodies may have introduced a bias in the binding
activity of these reference preparations towards more stable antibodies of
unknown clinical relevance. Due to the scarcity of these preparations, they are
only used as primary standards for calibrating assay methods. Commercial kits
contain secondary standards that differ for each method. Currently, assay
calibrations vary with the experimental conditions as well as the antigen
preparation used by the manufacturer. This may introduce additional bias in
detecting the heterogeneous antibodies present in patient specimens. In the case
of TRAb, the reference preparation MRC 90/672 is more recent (1990) but
currently used by few manufacturers.
TPOAb Measurements
Thyroid Peroxidase (TPO) is a 110 kD membrane bound hemo-glycoprotein with a
large extracellular domain, and a short transmembrane and intracellular domain.
TPO is involved in thyroid hormone synthesis at the apical pole of the
follicular cell. Several isoforms related to differential splicing of TPO RNA
have been described. TPO molecules may also differ with respect to their
three-dimensional structure, extent of glycosylation and heme binding. Most of
the TPO molecules do not reach the apical membrane and are degraded
intracellularly.
Guideline 32. Preferred TPOAb Methodology
- Sensitive, specific TPOAb immunoassays, using suitable preparations of
highly purified native or recombinant human TPO as the antigen, should replace
the older insensitive, semi-quantitative anti-microsomal antibody (AMA)
agglutination tests.
(Consensus Level 90%)
- The clinical significance of a low TPOAb concentration requires more
study.
TPO autoantibodies were initially described as anti-microsomal autoantibodies
(AMA) since they were found to react with crude preparations of thyroid cell
membranes. The microsomal antigen was later identified as TPO.[265]
Older AMA immunofluorescence assays as well as passive tanned red cell
agglutination tests are still currently in use in addition to the newer, more
sensitive competitive and non-competitive TPOAb immunoassays. These new
immunoassay methods are currently replacing the older AMA agglutination tests
because they are quantitative, more sensitive and can easily be automated.
However, there is wide variability in the sensitivity and specificity of these
new TPOAb methods. Some of this variability stems from differences in the TPO
preparations used in the various assay kits. When extracted from human thyroid
tissue, TPO may be used as a crude membrane preparation or may be purified by
different methods. The assay specificity may also differ because of
contamination by other thyroid antigens - notably Tg and/or variations in the
three-dimensional structure of TPO. The use of recombinant human TPO (rhTPO)
eliminates the risk of contamination but does not solve the problem of the
differences in TPO structure that depend upon the technique used to isolate TPO.
Most current TPOAb assays are quantitated in international units using the
reference preparation MRC 66/387. Unfortunately, the use of this primary
standard does not alleviate between-method variations as is evident from the
broad variability in sensitivity limits claimed by the different kit
manufacturers (range <0.3 to <20 kIU/L) and the differences in normal
reference intervals.
TPOAb Prevalence & Reference Intervals
The estimate of TPOAb prevalence depends on the sensitivity and specificity
of the method employed. The recent NHANES III United States survey of ~17,000
subjects without apparent thyroid disease, reported detectable TPOAb levels in
12 % of subjects using a competitive immunoassay method.[18] Whether
low levels of TPOAb detected in healthy individuals and/or patients with
non-thyroid autoimmune diseases reflect normal physiology, the prodrome of AITD,
or an assay specificity problem, remains unclear.
Normal reference values for TPOAb assays are highly variable and often
arbitrarily established, so that a large majority of patients with AITD test
positive, and most subjects without clinical evidence of AITD test negative. The
lower normal limit appears to relate to technical factors. Specifically, assays
citing a low detection limit (<10 kIU/L) typically report undetectable TPOAb
levels in meticulously selected normal subjects. Such methods suggest that the
presence of TPOAb is a pathologic finding. In contrast, TPOAb assays reporting
higher detection limits (>10kIU/L) typically cite a TPOAb "normal reference
range". Since such methods appear to have no enhanced sensitivity for detecting
AITD, these "normal range" values may represent non-specific assay "noise" and
may not be pathologically meaningful.
The recent 20-year follow-up study of the Whickham cohort reported that
detectable TPOAb titers (measured as AMA) was not only a risk factor for
hypothyroidism but that a detectable AMA preceded the development of an elevated
TSH (Figure 5).[35] This suggests that a detectable TPOAb is a risk
factor for AITD (Guideline 34). However, individuals with low TPOAb levels would
have had undetectable AMA by the older methods used in this
study.[35] Indeed, AMA-negative subjects with TSH >2 mIU/L did
have a higher long-term risk of hypothyroidism, suggesting that low TPOAb levels
may be clinically significant.[35] Thus, whether individuals with low
levels of TPOAb and/or TgAb should be considered normal remains in question
until more long-term follow-up studies on such individuals show that they do not
have an increased risk for developing thyroid dysfunction.
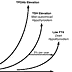 |
Figure 5. (click image to zoom) TPOAb Changes with
Developing Autoimmune Thyroid Dysfunction
|
Guideline 33. Reference Intervals for Thyroid Antibody Tests
Reference intervals for thyroid antibody tests should be established from
120 "Normal" subjects free from any history of thyroid disease: Subject
selection should minimize the inclusion of persons with a predisposition for
autoimmune thyroid disease. Normal subjects should be:
-
Male
-
Young (< 30 years of age)
-
Have serum TSH levels between 0.5 and 2.0 mIU/L
-
No goiter
-
No personal or family history of thyroid disease
-
No non-thyroid autoimmune diseases (e.g. lupus or
diabetes)
The criteria employed for selecting subjects for the normal cohort used to
establish an autoantibody normal reference range, is critical. Such a cohort
should be comprised of young, biochemically euthyroid (TSH 0.5 to 2.0 mIU/L)
male subjects with no goiter and no family history of AITD. This rigorous
selection process would be least likely to include subjects with a
predisposition to AITD.
Clinical Uses of TPOAb Measurements
TPOAb is the most sensitive test for detecting autoimmune thyroid
disease.[266] As shown schematically in Figure 5, TPOAb is typically
the first abnormality to appear in the course of developing hypothyroidism
secondary to Hashimotos' thyroiditis. In fact, when TPOAb is measured by a
sensitive immunoassay, >95% of subjects with Hashimotos thyroiditis have
detectable levels of TPOAb. Such methods also detect TPOAb in most (~85%)
patients with Graves' disease.[254] Patients with TPOAb detected in
early pregnancy are at risk for developing post-partum
thyroiditis.[50] Patients with Down's syndrome have an increased risk
of thyroid dysfunction due to autoimmune thyroid disease and annual screening
with TSH and TPOAb is important.[267,268]
Recent reports have suggested that the IQ of children born to mothers with
increased TSH and/or detectable TPOAb during pregnancy may be
compromised.[63-65] This has prompted recommendations that all
pregnant women should have TSH and TPOAb levels measured in the first trimester
of their pregnancy [Section-2 A3 and Guideline 4]. Further, TPOAb measurements
may have a role in infertility, since high TPOAb levels are associated with a
high risk of miscarriage and failure to conceive with in-vitro
fertilization.[269]
Guideline 34. Recommended Uses for TPOAb Measurement
-
Diagnosis of Autoimmune Thyroid Disease
-
Risk factor for Autoimmune Thyroid Disease
-
Risk factor for hypothyroidism during Interferon alpha, Interleukin-2 or
Lithium therapy
-
Risk factor for thyroid dysfunction during amiodarone therapy (see Guideline
5)
-
Risk factor for hypothyroidism in Down's Syndrome patients
-
Risk factor for thyroid dysfunction during pregnancy and for post-partum
thyroiditis
-
Risk factor for miscarriage and in-vitro fertilization
failure
The presence of TPOAb is well established as a risk factor for thyroid
dysfunction when patients are being treated with lithium, amiodarone,
interleukin-2 or interferon-alpha.[75,259,260,261,270] During
interferon-alpha treatment, a preexisting thyroid autoimmune disorder or
detectable TPOAb titer are predisposing factors for the development of thyroid
disease during therapy.[262] There appears however, to be no
increased frequency of thyroid dysfunction during interferon-beta
therapy.[271] The presence of TPOAb before therapy shows a
sensitivity of 20%, a specificity of 95% and a predictive value of 66.6% for the
development of thyroid dysfunction.[272]
Thyroglobulin Autoantibody (TgAb) Measurements
Thyroglobulin (Tg), the prothyroid globulin, is a high molecular weight (660
kDa) soluble glycoprotein made up of two identical subunits. Tg is present with
a high degree of heterogeneity due to differences in post-translational
modifications (glycosylation, iodination, sulfation etc). During the process of
thyroid hormone synthesis and release, Tg is polymerized and degraded.
Consequently, the immunologic structure of Tg is extremely complex. The
characteristics of Tg preparations may vary widely depending on the starting
human thyroid tissue and the purification process used. This is the first clue
to explain why TgAb assays, as well as Tg assays [Section-3 E2] are so difficult
to standardize.
TgAb Methodology
As with TPOAb methods, the design of TgAb assays has evolved from
immunofluorescence of thyroid tissue sections, to passive tanned red cell
agglutination methods and now to the competitive and noncompetitive
immunoassays. This technical evolution has improved both the sensitivity and
specificity of serum TgAb measurements. However, because the older and newer
methods are still being used concurrently in clinical laboratories, the
sensitivity and specificity of available methods can vary widely depending on
the laboratory. Assays are calibrated with purified or crude preparations of
TgAb by pooling patient sera or blood donor material. These various secondary
standards are often, but not always, calibrated against the primary standard
(MRC 65/93). However, standardization with MRC 65/93 does not ensure that
different methods are quantitatively or qualitatively similar. Other reasons for
method differences relate to the heterogeneity of TgAb itself. The heterogeneity
of TgAb is restricted in patients with AITD compared with other thyroid
disorders such as differentiated thyroid carcinomas (DTC) in which the
heterogeneity of TgAb appears less restricted.[273] This reflects
differences in the expression of the different autoantibodies that may be
normally expressed at very low levels in healthy individuals.[274]
The inter-method variability of serum TgAb values may also reflect qualitative
differences in TgAb affinity and epitope specificity in different serum samples
from patients with different underlying thyroid and immunological conditions.
Another reason for inter-method differences is that assay designs are prone to
interference by high levels of circulating antigen (Tg), as is commonly the case
with Graves' disease and metastatic DTC.[275]
Guideline 35. For Manufacturers Developing TgAb Methods
TgAb Prevalence & Reference Intervals
As with TPO antibodies, the prevalence and normal cut-off values for
thyroglobulin antibodies depends on the sensitivity and specificity of the assay
method.[276] The NHANES III survey reported a TgAb prevalence of ~10%
for the general population, measured by competitive immunoassay.[18]
The TgAb prevalence in DTC patients appears to be two-fold higher than the
normal population (~20 versus 10 %, respectively).[276] As with
TPOAb, the clinical significance of low TgAb levels, that would be undetectable
by the older agglutination methods, remains unclear. It has been suggested that
low levels may represent " natural " antibody in normal individuals or a "
scavenger " antibody response to antigen release following thyroid surgery or
radioactive iodide therapy. Alternatively, low levels might represent underlying
silent AITD.[256] Different TgAb methods report different normal
threshold values, as discussed for TPOAb [Section-3 D5(a)]. Specifically, some
TgAb methods report that normal subjects should have values below the assay
detection level, other methods report a "normal range". When TgAb measurements
are used as an adjunct test to serum Tg measurements, the significance of low
TgAb levels relates less to the pathophysiology of its presence but more to the
potential for low TgAb levels to interfere with the serum Tg method.
Guideline 36. TgAb Measurement in Non-Neoplastic Conditions
-
In iodide sufficient areas, it is not usually necessary or
cost-effective to order both TPOAb and TgAb, because TPOAb-negative patients
with detectable TgAb rarely display thyroid dysfunction.
-
In iodide deficient areas, serum TgAb measurements may be useful for
detecting autoimmune thyroid disease when patients have a nodular goiter.
-
Monitoring iodide therapy for endemic goiter.
Sensitivity and Precision of TgAb Measurement
Sensitive quantitative TgAb determination is a critical adjunct test for
serum Tg measurement. Qualitative agglutination tests are not sufficiently
sensitive to detect the low TgAb concentrations that can interfere with serum Tg
measurements.[276] As with TPOAb assays [Section-3 D5(a)], the
absolute values reported by different TgAb immunoassays are highly variable
which precludes the use of different manufacturers tests for serial monitoring
of DTC patients. There appear to be two classes of TgAb immunoassay. One class
is characterized by low detection limits (<10 kIU/L) and an undetectable
normal reference limit. Such methods suggest that the presence of TgAb is a
pathologic finding. The other class of assay reports higher detection limits
(>10kIU/L) and cites a TgAb "normal reference range". These detectable
"normal range" values are likely to represent non-specific assay "noise" caused
by assay insensitivity or problems with specificity since these low "normal
range" values show no evidence of interference with serum Tg measurements
[Section-3 E6].
Guideline 37. TgAb Measurement in Differentiated Thyroid Carcinomas
(DTC)
The TgAb concentration should be measured in ALL patient sera prior
to Tg analysis because low levels of TgAb can interfere with serum Tg
measurements causing either falsely low, undetectable or high values depending
on the Tg method used.
-
TgAb should be measured in every serum specimen sent to the laboratory for Tg
testing.
-
Serial TgAb measurements should be made on all TgAb-positive DTC patients
using the same manufacturer's method because serial TgAb values have prognostic
significance for monitoring response to DTC treatment.
-
TgAb methods should be immunoassay not agglutination, because low levels of
TgAb can interfere with serum Tg measurements made by most methods, and serial
measurements must be quantitative not qualitative.
-
Serum Tg recovery tests do not reliably detect the presence of TgAb and
should be discouraged as a method for detecting TgAb (Guideline 46).
-
Before changing the TgAb method, the laboratory should inform physician users
and evaluate the relationship between the old and proposed new method values.
Patients should be re-baselined if the difference between the methods is >10%
CV.
Clinical Uses of TgAb Measurement
There is some debate over the clinical utility of serum TgAb measurement for
assessing the presence of thyroid autoimmunity. The United States NHANES III
study reported that 3 % of subjects with no risk factors for thyroid disease had
detectable TgAb without associated presence of TPOAb.[18] Since this
cohort had no associated TSH elevation, TgAb measurements do not appear to be a
useful diagnostic test for AITD in areas of iodide
sufficiency.[256,279] In iodide deficient areas however, TgAb is
believed to be useful for detecting AITD, especially for patients with a nodular
goiter. TgAb measurements are also useful for monitoring iodide therapy for
endemic goiter, since iodinated Tg molecules are more immunogenic.
Serum TgAb testing is primarily used as an adjunct test when serum Tg
measurements are requested. The clinical utility of TgAb measurements in sera
from DTC patients is two-fold. First, sensitive and specific TgAb screening of
sera in these cancer patients is necessary, because even low antibody
concentrations can interfere with the Tg measurements made by most Tg methods
[see Section-3 E6].[275,276] Second, serial TgAb measurements
themselves may serve as a surrgogate tumor marker test for TgAb-positive
patients in whom Tg testing may be unreliable.[276] Specifically,
TgAb-positive patients who are rendered disease-free typically become
TgAb-negative within 1-4 years.[276,277,278] In contrast, patients
who have persistent disease after treatment retain detectable TgAb
concentrations. In fact, a rise in the TgAb level is often the first indication
of recurrence in such patients.[276]
TSH Receptor Autoantibodies (TRAb)
The TSH receptor is a member of the superfamily of receptors with seven
transmembrane domains linked to G proteins. The 60kb TSH receptor gene located
on the long arm of chromosome 14q31 has been cloned and
sequenced.[272] Exons 1-9 code for the extracellular domain of the
receptor (397 amino acids) and exon 10 codes for the transmembrane region (206
amino acids). Activation of G proteins by the hormone receptor complex results
in stimulation of cAMP production by adenylate cyclase and inositol phosphate
turnover by phospholipases.[280] Site-directed mutagenesis has shown
that the 3-dimensional receptor structure is important for the interaction with
TSH and/or TRAbs. There are three broad types of TRAb measured by either
bioassay or receptor assay (Table 6). Receptor, or TSH Binding Inhibitory Immunoglobulin
(TBII) assays do not measure biologic activity directly but assess whether the
specimen contains immunoglobulins that can block the binding of TSH to an in
vitro receptor preparation. TSH stimulating antibodies (TSAb) appear to bind the
N-terminal portion of the extracellular domain and mimic the actions of TSH by
inducing post-receptor signal transduction and cell stimulation. In contrast,
the C-terminal region is more important for TSH receptor blocking antibodies
(abbreviated TBAb or TSBAb) which block stimulation by either TSAb or TSH,
causing hypothyroidism.[281] Thyroid growth-stimulating
immunoglobulins (TGI) are less well characterized in this regard.
It has now been shown that the lack of correlation between TRAb levels and
the clinical status of patients is largely because circulating TRAb's are
heterogeneous. The fact that TRAb heterogeneity can coexist within an individual
patient and change over time is one reason why it has been difficult to develop
diagnostically accurate TRAb tests.[282,283] Indeed, the clinical
presentation of Graves' patients who exhibit both TSAb and TBAb/TSBAb will
likely depend on the relative concentration and affinity of the predominant
antibody. A shift from stimulating to blocking TRAb may explain the spontaneous
remission of Graves' disease during pregnancy as well as radioiodide induction
of transient hypothyroidism.[281,284] It is important to note that
bioassays that use cell preparations to measure the biologic effects of TRAb
(stimulation, inhibition of TSH activity or growth) can detect functional
changes in TRAb heterogeneity. In contrast, the receptor, or TSH Binding
Inhibitory Immunoglobulin (TBII) type of assays, which are used by many clinical
laboratories, merely measure the ability of a serum or IgG preparation to block
the binding of a TSH preparation and do not measure the biological response (Table 6). This fundamental difference in assay design explains
why bioassays and receptor assays usually display a weak correlation (r 5
0.31-0.65).[283,285]
TRAb Methodology
The first report that there was a thyroid stimulator that differed from TSH
with respect to its longer half-life (Long Acting Thyroid Stimulator or LATS)
was published in 1956 using an in vivo bioassay.[286] LATS was later
identified as an immunoglobulin. Like TSH, TRAbs stimulate both cAMP and the
inositol phosphate pathways of the thyroid follicular cell, and thus both
stimulate and block both thyroid hormone synthesis and the growth of the
gland.[283]
The types of methods developed for TRAb measurements are classified relative
to their functional activity, as shown in Table 6. Studies in mice and FRTL-5 cell lines as well as
humans, show that a high concentration of human chorionic gonadotropin (hCG) is
also a weak TRAb agonist and can stimulate cAMP, iodide transport, and cell
growth.[56] The marked hCG elevations secondary to choriocarcinoma
can in rare cases cause a false positive TRAb result. However, the increase in
hCG typically seen with normal pregnancy or in patients treated for a hydatiform
mole is usually not high enough to elicit a false positive result.
Bioassays (TSAb, TBAb/TSBAb and TGI)
Most current bioassays are based on TSH receptor activation of second
messenger (cAMP) production from a cell preparation (FRTL-5/ CHO TSH-R) exposed
to a serum specimen or IgG preparation.[287-289] The recent cloning
of the TSH receptor has benefited bioassays by facilitating the development of
TSH receptor transfected cell lines.[290,291] Although these
bioassays are available in several commercial laboratories in the United States
and Asia, they are less available in Europe because of regulations that affect
the use of genetically altered organisms. Unfortunately, the correlation between
TRAb assay results and clinical presentation is still poor. For example, the
diagnostic sensitivity for Graves' disease using TRAb bioassays ranges from 62.5
to 81%.[283] New approaches employing chimeric assays may be able to
target the loci of TRAb epitopes and TSH binding sites and thus provide a better
correlation between assay response and clinical
outcome.[281,284,292-294]
Receptor (TBII) Assays
Thyroid binding inhibiting immunoglobulin (TBII) assays are commercially
available and are used by many clinical laboratories. These methods quantify the
inhibition of the binding of 125I-labeled TSH to either solubilized porcine
receptors, or more recently, recombinant human TSH
receptors.[295-297] This type of method does not distinguish between
stimulating and blocking TRAbs. TBII activity is typically quantified against a
TRAb-positive serum calibrated against a reference calibrator serum. The most
frequently used calibrator serum has been the MRC reference serum, LATS-B. A WHO
standard (MRC 90/672) has recently become available. The inherent heterogeneity
of TRAb in patient serum and the source of receptors used (porcine versus
recombinant human) are likely causes for the wide variability observed between
TBII methods, despite the use of the same standard.[283,298] Although
TBII methods based on recombinant human TSH receptor are now available and may
have a higher diagnostic sensitivity for Graves' disease, they do not appear to
offer improved specificity or sensitivity for predicting response to
anti-thyroid drug (ATD) therapy.[297,299]
TRAb Reference Intervals
Guideline 38. TSH Receptor Antibody (TRAb) Tests
Clinical laboratory TRAb assays:
-
Receptor or TSH binding inhibition tests (TBII) that do not measure
stimulatory activity directly but detect factors in the serum specimen that
block the binding of a labeled TSH preparation to an in-vitro TSH receptor
preparation. These tests are the more commonly used TRAb assays in clinical
laboratories.
-
TSH receptor bioassays (TSAb) that use cells (FRTL-5 cells, or more recently
CHO transfected with human TSH receptor) to detect thyroid stimulating
immunoglobulins (TSAb) that either stimulate cAMP or iodide uptake. These tests
are not routinely available in all countries.
-
In general, there is a poor correlation between TSAb and TBII results
(60-75%). TSAb assays claim to be positive in 80-100% and TBII assays positive
in 70 to 90% of untreated Graves' hyperthyroid patients. Neither test has high
specificity or sensitivity for predicting remission from Graves'
hyperthyroidism.
-
Normal hCG as well as abnormal hCG production in choriocarcinoma are known to
interact with the TSH receptor which could lead to false positive results. This
might be observed in rare cases of choriocarcinoma but not in normal pregnancy
or treated hydatiform mole in which the level of hCG is not high enough to cause
a false positive result.
Despite the adoption of a new international reference preparation MRC 90/672,
TRAb values are still method-dependent and reference intervals vary depending on
the selection of the "normal" population used to determine the cut-off level for
a positive result. This cut-off is generally defined as two standard deviations
from the mean of normal subjects.
Clinical Uses of TRAb Measurement
The clinical use of TRAb measurements for the diagnosis and follow-up of AITD
remains a matter of controversy and differs geographically. The differential
diagnosis of hyperthyroidism can be resolved in most patients without resorting
to TRAb testing. Nevertheless, the presence of TRAb may distinguish Graves'
disease from factitious thyrotoxicosis and other manifestations of
hyperthyroidism such as subacute or post-partum thyroiditis and toxic nodular
goiter.
TRAb measurements have also been proposed as a means for predicting the
course of Graves' disease. A declining TRAb level is often seen in hyperthyroid
patients in clinical remission after treatment with antithyroid drugs (ATD).
After ATD withdrawal, very high levels of TRAb correlate quite well with prompt
relapse, but this situation involves very few patients. Conversely, a
significant number of patients with undetectable or low TRAb levels will
relapse. A meta-analysis of the relationship between TRAb levels and the risk of
relapse has shown that 25% of patients are misclassified by TRAb
assays.[263] This suggests that after ATD therapy, a follow-up of the
patients is necessary whatever the TRAb level at the time of ATD withdrawal and
that TRAb measurement is not cost effective for this
purpose.[263]
There is general agreement that TRAb measurements can be used to predict
fetal and/or neonatal thyroid dysfunction in pregnant women with a previous
history of AITD.[8,252] High levels of TRAb in the mother during the
third trimester of pregnancy suggest a risk of thyroid dysfunction in the
offspring.[8,282] Two to 10% of pregnant women with very elevated
TRAb deliver newborns with hyperthyroidism.[8] The risk for neonatal
hyperthyroidism is negligible following successful treatment of hyperthyroidism
with antithyroid drugs, but can develop after radioiodide treatment if TRAb
levels remain elevated.[8] Euthyroid pregnant women (+/- L-T4
treatment) who have had prior radioiodide therapy for Graves' disease should
have TRAb levels measured both in early pregnancy, when an elevated value is a
significant risk factor for fetal hyperthyroidism, and during the third
trimester, to evaluate for the risk of neonatal hyperthyroidism.[8]
Pregnant women who take antithyroid drugs (ATD) for Graves' disease should have
TRAb measured in the third trimester. High TRAb levels in such patients should
prompt a thorough clinical and biochemical evaluation of the neonate for
hyperthyroidism, both at birth (cord blood) and at 4 - 7 days, after the effects
of the transplacental passage of ATD have disappeared.[300] It is
worth noting that the TBII receptor assays are often used for this purpose since
they detect both stimulating (TSAb) and in rare cases, blocking antibodies
(TBAb/TSBAb) which cause transient hypothyroidism in 1:180,000 of
newborns.[301] It is also advisable to test for both stimulating and
blocking antibodies because the expression of thyroid dysfunction may be
different in the mother and the infant.[253]
Guideline 39. Clinical Uses of TRAb Measurement
-
To investigate the etiology of hyperthyroidism when the diagnosis is not
clinically obvious.
-
A declining TRAb concentration during long-term antithyroid drug therapy is
suggestive of remission. However TRAb measurements can be misleading in 25% of
such patients.
-
TRAb measurements are useful to diagnose Graves' disease patients and for
relating TRAb values to a treatment algorithm.
-
To evaluate patients suspected of "euthyroid Graves' opthalmopathy".
Undetectable TRAb however, does not exclude the condition.
-
Although TSAb assays have theoretical advantages, some believe that TBII
tests, that detect both stimulating (TSAb) and the rare cases of blocking
(TBAb/TSBAb) antibodies, are equally useful.
-
For pregnant women with a past or present history of Graves' disease. Note:
Pregnant women who are euthyroid after receiving prior antithyroid drug
treatment for Graves' disease have a negligible risk for fetal or neonatal
hyperthyroidism.
-
Euthyroid pregnant women (± L-T4 treatment) who have had prior radioiodide
treatment for Graves' disease should have TRAb measured both early in pregnancy
when a high value is a risk factor for fetal hyperthyroidism (2-10%), and during
the third trimester to evaluate the risk of neonatal hyperthyroidism.
-
Pregnant women who take antithyroid drugs (ATD) for Graves' disease to
maintain a euthyroid state during pregnancy should have TRAb measured in the
third trimester. A high TBII value should prompt a clinical and biochemical
evaluation of the neonate for hyperthyroidism, both at birth (cord blood) and at
4 - 7 days after the effects of transplacental passage of ATD have been
lost.
-
The assessment of the risk of fetal and neonatal thyroid dysfunction
necessitates the detection of either blocking or stimulating TRAb when mothers
have no intact thyroid following past therapy for Graves' hyperthyroidism.
-
To identify neonates with transient hypothyroidism due to the presence of TSH
receptor blocking antibodies.
Guideline 40. Improvements Needed in Thyroid Antibody Tests
-
Current thyroid autoantibody assays should be submitted to a comparative
study of their analytical and clinical performances.
-
A comparison study of the antigen preparations currently in use would
facilitate the identification of the method(s) best suited for clinical thyroid
autoantibody testing.
-
The characteristics of the antigen preparations used in the test should be
stated for all thyroid autoantibody assays.
-
Reference preparations of antigens should be made
available.
The role of TRAb in thyroid-associated opthalmopathy (TAO) is
uncertain.[302] TAO appears to be exacerbated by radioiodide
therapy.[303] Furthermore, TRAb and other thyroid antibody levels
increase significantly after radioiodide therapy.[304-306] This
suggests that TRAb measurements prior to radioiodide therapy may be useful to
predict the risk of TAO but as yet there are no prospective studies to document
this observation.
Future Directions
It is important that a well-structured comparative study of the commercially
available thyroid autoantibody assays be performed. This would provide
irrefutable evidence that differences exist in the performance of current assay
methods.[296] It would also help to convince clinical laboratory
scientists to avoid using assays that have poor clinical performance and
encourage manufacturers to improve their products or drop them from the
market.
Guideline 41. For Manufacturers Developing Thyroid Antibody Tests
-
Absolute or "gold standard" methods remain a target for the future.
-
The kit package insert should document the methods used to produce the
antigen reagents, the assay design and all experimental conditions affecting the
antigen-antibody interactions.
-
The specificity of the secondary standards should be selected relative to the
interactions between the autoantibodies in patient sera and their specific
antigen.
-
TPOAb and TgAb IMAs should be checked for hook effects using ~20 specimens
with antibody concentrations >1,000 kIU/L and ~20 specimens with values above
10,000 kIU/L.
-
TgAb methods should be checked for high antigen (Tg) effects by spiking a
range of sera containing low TgAb concentration to Tg levels >10,000 µg/L
(ng/ml) and >100,000 µg/L (ng/ml).
References
- Nohr SB, Laurberg P, Borlum KG, Pedersen Km, Johannesen PL, Damm P. Iodine
deficiency in pregnancy in Denmark. Regional variations and frequency of
individual iodine supplementation. Acta Obstet Gynecol Scand 1993;72:350-3.
- Glinoer D. Pregnancy and iodine. Thyroid 2001;11:471-81.
- Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter EW, Maberly GF et
al. Iodine nutrition in the Unites States. Trends and public health
implications: iodine excretion data from National Health and Nutrition
Examination Surveys I and III (1971-1974 and 1988-1994). J Clin Endocrinol Metab
1998;83:3398-400.
- Wartofsky L, Glinoer D, Solomon d, Nagataki S, Lagasse R, Nagayama Y et al.
Differences and similarities in the diagnosis and treatment of Graves disease in
Europe, Japan and the United States. Thyroid 1990;1:129-35.
- Singer PA, Cooper DS, Levy EG, Ladenson PW, Braverman LE, Daniels G et al.
Treatment guidelines for patients with hyperthyroidism and hypothyroidism. JAMA
1995;273:808-12.
- Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG et al.
Treatment Guidelines for Patients with Thyroid Nodules and Well-differentiated
Thyroid Cancer. Arch Intern Med 1996;156:2165-72.
- Vanderpump MPJ, Ahlquist JAO, Franklyn JA and Clayton RN. Consensus
statement for good practice and audit measures in the management of
hypothyroidism and hyperthyroidism. Br Med J 1996;313:539-44.
- Laurberg P, Nygaard B, Glinoer D, Grussendorf M and Orgiazzi J. Guidelines
for TSH-receptor antibody measurements in pregnancy: results of an
evidence-based symposium organized by the European Thyroid Association. Eur J
Endocrinol 1998;139:584-6.
- Cobin RH, Gharib H, Bergman DA, Clark OH, Cooper DS, Daniels GH et al.
AACE/AAES Medical/Surgical Guidelines for Clinical Practice: Management of
Thyroid Carcinoma. Endocrine Pract 2001;7:203-20.
- Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG et al. American
Thyroid Association Guidelines for detection of thyroid dysfunction. Arch Intern
Med 2000;160:1573-5.
- Brandi ML, Gagel RJ, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C et al.
Consensus Guidelines for Diagnosis and Therapy of MEN Type 1 and Type 2. J Clin
Endocrinol Metab 2001;86:5658-71.
- Werner and Ingbar's "The Thyroid". A Fundamental and Clinical Text.
Lippincott-Raven, Philadelphia 2000. Braverman LE and Utiger RD eds.
- DeGroot LJ, Larsen PR, Hennemann G, eds. The Thyroid and Its Diseases.
(www.thyroidmanager.org) 2000.
- Piketty ML, D'Herbomez M, Le Guillouzic D, Lebtahi R, Cosson E, Dumont A et
al. Clinical comparision of three labeled-antibody immunoassays of free
triiodothyronine. Clin Chem 1996;42:933-41.
- Sapin R, Schlienger JL, Goichot B, Gasser F and Grucker D. Evaluation of the
Elecsys free triiodothyronine assay; relevance of age-related reference ranges.
Clin Biochem 1998;31:399-404.
- Robbins J. Thyroid hormone transport proteins and the physiology of hormone
binding. In "Hormones in Blood". Academic Press, London 1996. Gray CH, James
VHT, eds. pp 96-110.
- Demers LM. Thyroid function testing and automation. J Clin Ligand Assay
1999;22:38-41.
- Hollowell JG, Staehling NW, Hannon WH, Flanders WD, Gunter EW, Spencer CA et
al. Serum thyrotropin, thyroxine and thyroid antibodies in the United States
population (1988 to 1994):|NHANES III. J Clin Endocrinol Metab 2002;87:489-99.
- Wardle CA, Fraser WD and Squire CR. Pitfalls in the use of thyrotropin
concentration as a first-line thyroid-function test. Lancet 2001;357:1013-4.
- Spencer CA, LoPresti JS, Patel A, Guttler RB, Eigen A, Shen D et al.
Applications of a new chemiluminometric thyrotropin assay to subnormal
measurement. J Clin Endocrinol Metab 1990;70:453-60.
- Meikle, A. W., J. D. Stringham, M. G. Woodward and J. C. Nelson. Hereditary
and environmental influences on the variation of thyroid hormones in normal male
twins. J Clin Endocrinol Metab1988;66:588-92.
- Andersen S, Pedersen KM, Bruun NH and Laurberg P. Narrow individual
variations in serum T4 and T3 in normal subjects: a clue to the understanding of
subclinical thyroid disease. J Clin Endocrinol Metab 2002;87:1068-72.
- Cooper, D. S., R. Halpern, L. C. Wood, A. A. Levin and E. V. Ridgway.
L-thyroxine therapy in subclinical hypothyroidism. Ann Intern Med
1984;101:18-24.
- Biondi B, Fazio E, Palmieri EA, Carella C, Panza N, Cittadini A et al. Left
ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J
Clin Endocrinol Metab 1999;2064-7.
- Hak AE, Pols HAP, Visser TJ, Drexhage HA, Hofman A and Witteman JCM.
Subclinical Hypothyroidism is an independent risk factor for atherosclerosis and
myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med
2000;132:270-8.
- Michalopoulou G, Alevizaki M, Piperingos G, Mitsibounas D, Mantzos E,
Adamopoulos P et al. High serum cholesterol levels in persons with 'high-normal'
TSH levels: should one extend the definition of subclinical hypothyroidism? Eur
J Endocrinol 1998;138:141-5.
- Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC and Weintraub BD.
Thyrotropin-secreting pituitary tumors. Endocrine Rev 1996;17:610-38.
- Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL and Weintraub BD.
Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone
sensitivity and treatment outcome in 25 patients followed at the National
Institutes of Health. J Clin Endocrinol Metab 76 1999;:1089-94.
- Oliveira JH, Persani L, Beck-Peccoz P and Abucham J. Investigating the
paradox of hypothyroidism and increased serum thyrotropin (TSH) levels in
Sheehan's syndrome: characterization of TSH carbohydrate content and
bioactivity. J Clin Endocrinol Metab 2001;86:1694-9.
- Uy H, Reasner CA and Samuels MH. Pattern of recovery of the
hypothalamic-pituitary thyroid axis following radioactive iodine therapy in
patients with Graves' disease. Amer J Med 1995;99:173-9.
- Hershman JM, Pekary AE, Berg L, Solomon DH and Sawin CT. Serum thyrotropin
and thyroid hormone levels in elderly and middle-aged euthyroid persons. J Am
Geriatr Soc 1993;41:823-8.
- Fraser CG. Age-related changes in laboratory test results. Clinical
applications. Drugs Aging1993;3:246-57.
- Fraser CG. 2001. Biological Variation: from principles to practice. AACC
Press, Washington DC.
- Drinka PJ, Siebers M and Voeks SK. Poor positive predictive value of low
sensitive thyrotropin assay levels for hyperthyroidism in nursing home
residents. South Med J 1993;86:1004-7.
- Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Rodgers H et
al. The incidence of thyroid disorders in the community; a twenty year follow up
of the Whickham survey. Clin Endocrinol 1995;43:55-68.
- Sawin CT, Geller A, Kaplan MM, Bacharach P, Wilson PW, Hershman JM et al.
Low serum thyrotropin (thyroid stimulating hormone) in older persons without
hyperthyroidism. Arch Intern Med1991;151:165-8.
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P and Franklyn JA. Prediction of
all-cause and cardiovascular mortality in elderly people from one low serum
thyrotropin result: a 10-year study. Lancet 2001;358:861-5.
- Nelson JC, Clark SJ, Borut DL, Tomei RT and Carlton EI. Age-related changes
in serum free thyroxine during childhood and adolescence. J Pediatr
1993;123:899-905.
- Adams LM, Emery JR, Clark SJ, Carlton EI and Nelson JC. Reference ranges for
newer thyroid function tests in premature infants. J Pediatr 1995;126:122-7.
- Lu FL, Yau KI, Tsai KS, Tang JR, Tsao PN and Tsai WY. Longitudinal study of
serum free thyroxine and thyrotropin levels by chemiluminescent immunoassay
during infancy. T'aiwan Erh K'o i Hseh Hui Tsa Chih 1999;40:255-7.
- Zurakowski D, Di Canzio J and Majzoub JA. Pediatric reference intervals for
serum thyroxine,triiodothyronine, thyrotropin and free thyroxine. Clin Chem
1999;45:1087-91.
- Fisher DA, Nelson JC, Carlton Ei and Wilcox RB. Maturation of human
hypothalamic-pituitary-thyroid function and control. Thyroid 2000;10:229-34.
- Fisher DA, Schoen EJ, La Franchi S, Mandel SH, Nelson JC, Carlton EI and
Goshi JH. The hypothalamic-pituitary-thyroid negative feedback control axis in
children with treated congenital hypothyroidism. J Clin Endocrinol Metab
2000;85:2722-7.
- Penny R, Spencer CA, Frasier SD and Nicoloff JT. Thyroid stimulating hormone
(TSH) and thyroglobulin (Tg) levels decrease with chronological age in children
and adolescents. J Clin Endocrinol Metab 1983;56:177-80.
- Verheecke P. Free triiodothyronine concentration in serum of 1050 euthyroid
children is inversely related to their age. Clin Chem 1997;43:963-7.
- Glinoer D, De Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A et
al. Regulation of maternal thyroid function during pregnancy. J Clin Endocrinol
Metab 1990;71:276-87.
- Glinoer D. The regulation of thyroid function in pregnancy: pathways of
endocrine adaptation from physiology to pathology. Endocrinol Rev
1997;18:404-33.
- Weeke J, Dybkjaer L, Granlie K, Eskjaer Jensen S, Kjaerulff E, Laurberg P et
al. A longitudinal study of serum TSH and total and free iodothyronines during
normal pregnancy. Acta Endocrinol1982;101:531-7.
- Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS
et al. Amelioration of some pregnancy associated variation in thyroid function
by iodine supplementation. J Clin Endocrinol Metab 1993;77:1078-83.
- Nohr SB, Jorgensen A, Pedersen KM and Laurberg P. Postpartum thyroid
dysfunction in pregnant thyroid peroxidase antibody-positive women living in an
area with mild to moderate iodine deficiency:Is iodine supplementation safe? J
Clin Endocrinol Metab 2000;85:3191-8.
- Panesar NS, Li CY and Rogers MS. Reference intervals for thyroid hormones in
pregnant Chinese women. Ann Clin Biochem 2001;38:329-32.
- Nissim M, Giorda G, Ballabio M, D'Alberton A, Bochicchio D, Orefice R et al.
Maternal thyroid function in early and late pregnancy. Horm Res 1991;36:196-202.
- Talbot JA, Lambert A, Anobile CJ, McLoughlin JD, Price A, Weetman AP et al.
The nature of human chorionic gonadotropin glycoforms in gestational
thyrotoxicosis. Clin Endocrinol 2001;55:33-9.
- Jordan V, Grebe SK, Cooke RR, Ford HC, Larsen PD, Stone PR et al. Acidic
isoforms of chorionic gonadotrophin in European and Samoan women are associated
with hyperemesis gravidarum and may |be thyrotrophic. Clin Endocrinol
1999;50:619-27.
- Goodwin TM, Montoro M, Mestman JH, Pekary AE and Hershman JM. The role of
chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J
Clin Endocrinol Metab1992;75:1333-7.
- Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis
gravidarum and trophoblastic tumors. Thyroid 1999;9:653-7.
- McElduff A. Measurement of free thyroxine (T4) in pregnancy. Aust NZ J Obst
Gynecol 1999;39:158-61.
- Christofides, N., Wilkinson E, Stoddart M, Ray DC and Beckett GJ. Assessment
of serum thyroxine binding capacity-dependent biases in free thyroxine assays.
Clin Chem 1999;45:520-5.
- Roti E, Gardini E, Minelli R, Bianconi L, Flisi M,. Thyroid function
evaluation by different commercially available free thyroid hormone measurement
kits in term pregnant women and their newborns. J Endocrinol Invest 1991;14:1-9.
- Stockigt JR. Free thyroid hormone measurement: a critical appraisal.
Endocrinol Metab Clin N Am2001;30:265-89.
- Mandel SJ, Larsen PR, Seely EW and Brent GA. Increased need for thyroxine
during pregnancy in women with primary hypothyroidism. NEJM 1990;323:91-6.
- Burrow GN, Fisher DA and Larsen PR. Maternal and fetal thyroid function. N
Engl J Med1994;331:1072-8.
- Pop VJ, De Vries E, Van Baar AL, Waelkens JJ, De Rooy HA, Horsten M et al.
Maternal thyroid peroxidase antibodies during pregnancy: a marker of impaired
child development? J Clin Endocrinol Metab 1995;80:3561-6.
- Haddow JE, Palomaki GE, Allan WC, K. G. Williams JR, Gagnon J, O'Heir CE et
al. Maternal thyroid deficiency during pregnancy and subsequent
neuropsychological development of the child. NEJM1999;341:549-55.
- Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ et
al. Low maternal free thyroxine concentrations during early pregnancy are
associated with impaired psychomotor development in infancy. Clin Endocrinol
1999;50:147-8.
- Radetti G, Gentili L, Paganini C, Oberhofer R, Deluggi I and Delucca A.
Psychomotor and audiological assessment of infants born to mothers with
subclinical thyroid dysfunction in early pregnancy. Minerva Pediatr
2000;52:691-8.
- Surks MI and Sievert R. Drugs and thyroid function. NEJM 1995;333:1688-94.
- Kailajarvi M, Takala T, Gronroos P, Tryding N, Viikari J, Irjala K et al.
Reminders of drug effects on laboratory test results. Clin Chem
2000;46:1395-1400.
- Brabant A, Brabant G, Schuermeyer T, Ranft U, Schmidt FW, Hesch RD et al.
The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol
1989;121:95-100.
- Samuels MH and McDaniel PA. Thyrotropin levels during hydrocortisone
infusions that mimic fasting-induced cortisol elevations: a clinical research
center study. J Clin Endocrinol Metab1997;82:3700-4.
- Kaptein EM, Spencer CA, Kamiel MB and Nicoloff JT. Prolonged dopamine
administration and thyroid hormone economy in normal and critically ill
subjects. J Clin Endocrinol Metab 1980;51:387-93.
- Geffner DL and Hershman JM. Beta-adrenergic blockade for the treatment of
hyperthyroidism. Am J Med 1992;93:61-8.
- Meurisse M, Gollogly MM, Degauque C, Fumal I, Defechereux T and Hamoir E.
Iatrogenic thyrotoxicosis: causal circumstances, pathophysiology and principles
of treatment- reviw of the literature. World J Surg 2000;24:1377-85.
- Martino E, Aghini-Lombardi F, Mariotti S, Bartelena L, Braverman LE and
Pinchera A. Amiodarone:a common source of iodine-induced thyrotoxicosis. Horm
Res 1987;26:158-71.
- Martino E, Bartalena L, Bogazzi F and Braverman LE. The effects of
amiodarone on the Thyroid. Endoc Rev 2001;22:240-54.
- Daniels GH. Amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab
2001;86:3-8.
- Harjai KJ and Licata AA. Effects of amiodarone on thyroid function. Ann
Intern Med 1997;126:63-73.
- Caron P. Effect of amiodarone on thyroid function. Press Med
1995;24:1747-51.
- Bartalena L, Grasso L, Brogioni S, Aghini-Lombardi F, Braverman LE and
Martino E. Serum interleukin-6 in amiodarone-induced thyrotoxicosis. J Clin
Endocrinol Metab 1994;78:423-7.
- Eaton SE, Euinton HA, Newman CM, Weetman AP and Bennet WM. Clinical
experience of amiodarone-induced thyrotoxicosis over a 3-year period: role of
colour-flow Doppler sonography. Clin Endocrinol 2002;56:33-8.
- Lazarus JH. The effects of lithium therapy on thyroid and
thyrotropin-releasing hormone. Thyroid1998;8:909-13.
- Kusalic M and Engelsmann F. Effect of lithium maintenance therapy on thyroid
and parathyroid function. J Psych Neurosci 1999;24:227-33.
- Oakley PW, Dawson AH and Whyte IM. Lithium: thyroid effects and altered
renal handling. Clin Toxicol 2000;38:333-7.
- Mendel CM, Frost PH, Kunitake ST and Cavalieri RR. Mechanism of the
heparin-induced increase in the concentration of free thyroxine in plasma. J
Clin Endocrinol Metab 1987;65:1259-64.
- Iitaka M, Kawasaki S, Sakurai S, Hara Y, Kuriyama R, Yamanaka K et al. Serum
substances that interfere with thyroid hormone assays in patients with chronic
renal failure. Clin Endocrinol1998;48:739-46.
- Bowie LJ, Kirkpatrick PB and Dohnal JC. Thyroid function testing with the
TDx: Interference from endogenous fluorophore. Clin Chem 1987;33:1467.
- DeGroot LJ and Mayor G. Admission screening by thyroid function tests in an
acute general care teaching hospital. Amer J Med 1992;93:558-64.
- Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal
failure. Endocrinol Rev 1996;17:45-63.
- Van den Berghe G, De Zegher F and Bouillon R. Acute and prolonged critical
illness as different neuroendocrine paradigms. J Clin Endocrinol Metab
1998;83:1827-34.
- Van den Berhe G. Novel insights into the neuroendocrinology of critical
illness. Eur J Endocrinol2000;143:1-13.
- Wartofsky L and Burman KD. Alterations in thyroid function in patients with
systemic illness: the "euthyroid sick syndrome". Endocrinol Rev 1982;3:164-217.
- Spencer CA, Eigen A, Duda M, Shen D, Qualls S, Weiss S et al. Sensitive TSH
tests - specificity limitations for screening for thyroid disease in
hospitalized patients. Clin Chem 1987;33:1391-1396.
- Stockigt JR. Guidelines for diagnosis and monitoring of thyroid disease:
nonthyroidal illness. Clin Chem 1996;42:188-92.
- Nelson JC and Weiss RM. The effects of serum dilution on free thyroxine (T4)
concentration in the low T4 syndrome of nonthyroidal illness. J Clin Endocrinol
Metab 1985;61:239-46.
- Chopra IJ, Huang TS, Beredo A, Solomon DH, Chua Teco GN. Serum thyroid
hormone binding inhibitor in non thyroidal illnesses. Metabolism 1986;35:152-9.
- Wang R, Nelson JC and Wilcox RB. Salsalate administration - a potential
pharmacological model of the sick euthyroid syndrome. J Clin Endocrinol Metab
1998;83:3095-9.
- Sapin R, Schliener JL, Kaltenbach G, Gasser F, Christofides N, Roul G et al.
Determination of free triiodothyronine by six different methods in patients with
non-thyroidal illness and in patients treated with amiodarone. Ann Clin Biochem
1995;32:314-24.
- Docter R, van Toor H, Krenning EP, de Jong M and Hennemann G. Free thyroxine
assessed with three assays in sera of patients with nonthyroidal illness and of
subjects with abnormal concentrations of thyroxine-binding proteins. Clin Chem
1993;39:1668-74.
- Wilcox RB, Nelson JC and Tomei RT. Heterogeneity in affinities of serum
proteins for thyroxine among patients with non-thyroidal illness as indicated by
the serum free thyroxine response to serum dilution. Eur J Endocrinol
1994;131:9-13.
- Liewendahl K, Tikanoja S, Mahonen H, Helenius T, Valimaki M and Tallgren LG.
Concentrations of iodothyronines in serum of patients with chronic renal failure
and other nonthyroidal illnesses: role of free fatty acids. Clin Chem
1987;33:1382-6.
- Sapin R, Schlienger JL,Gasser F, Noel E, Lioure B, Grunenberger F.
Intermethod discordant free thyroxine measurements in bone marrow-transplanted
patients. Clin Chem 2000;46:418-22.
- Chopra IJ. Simultaneous measurement of free thyroxine and free
3,5,3'-triiodothyronine in undiluted serum by direct equilibriium
dialysis/radioimmunoassay: evidence that free triiodothyronine and free
thyroxine are normal in many patients with the low triiodothyronine syndrome.
Thyroid 1998;8:249-57.
- Hamblin PS, Dyer SA, Mohr VS, Le Grand BA, Lim C-F, Tuxen DB, Topliss DJ and
Stockigt JR. Relationship between thyrotropin and thyroxine changes during
recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol
Metab 1986;62:717-22.
- Brent GA and Hershman JM. Thyroxine therapy in patients with severe
nonthyroidal illnesses and low serum thyroxine concentrations. J Clin Endocrinol
Metab 1986;63:1-8.
- De Groot LJ. Dangerous dogmas in medicine: the nonthyroidal illness
syndrome. J Clin Endocrinol Metab 1999;84:151-64.
- Burman KD and Wartofsky L. Thyroid function in the intensive care unit
setting. Crit Care Clin2001;17:43-57.
- Behrend EN, Kemppainen RJ and Young DW. Effect of storage conditions on
cortisol, total thyroxine and free thyroxine concentrations in serum and plasma
of dogs. J Am Vet Med Assoc 1998;212:1564-8.
- Oddie TH, Klein AH, Foley TP and Fisher DA. Variation in values for
iodothyronine hormones,thyrotropin and thyroxine binding globulin in normal
umbilical-cord serum with season and duration of storage. Clin Chem
1979;25:1251-3.
- Koliakos G, Gaitatzi M and Grammaticos P. Stability of serum TSH
concentratin after non refriferated storage. Minerva Endocrinol 1999;24:113-5.
- Waite KV, Maberly GF and Eastman CJ. Storage conditions and stability of
thyrotropin and thyroid hormones on filter paper. Clin Chem 1987;33:853-5.
- Levinson SS. The nature of heterophilic antibodies and their role in
immunoassay interference. J Clin Immunoassay 1992;15:108-15.
- Norden AGM, Jackson RA, Norden LE, Griffin AJ, Barnes MA and Little JA.
Misleading results for immunoassays of serum free thyroxine in the presence of
rheumatoid factor. Clin Chem 1997;43:957-62.
- Covinsky M, Laterza O, Pfeifer JD, Farkas-Szallasi T and Scott MG. Lambda
antibody to Esherichia coli produces false-positive results in multiple
immunometric assays. Clin Chem 2000;46:1157-61.
- Martel J, Despres N, Ahnadi CE, Lachance JF, Monticello JE, Fink G,
Ardemagni A, Banfi G, Tovey J, Dykes P, John R, Jeffery J and Grant AM.
Comparative multicentre study of a panel of thyroid tests using different
automated immunoassay platforms and specimens at high risk of antibody
interference. Clin Chem Lab Med 2000;38:785-93.
- Howanitz PJ, Howanitz JH, Lamberson HV and Ennis KM. Incidence and mechanism
of spurious increases in serum Thyrotropin. Clin Chem 1982;28:427-31.
- Boscato, L. M. and M. C. Stuart. Heterophilic antibodies: a problem for all
immunoassays. Clin Chem1988;34:27-33.
- Kricka LJ. Human anti-animal antibody interference in immunological assays.
Clin Chem1999;45:942-56.
- Sapin R and Simon C. False hyperprolactinemia corrected by the use of
heterophilic antibody-blocking agent. Clin Chem 2001;47:2184-5.
- Feldt-Rasmussen U, Petersen PH, Blaabjerg O and Horder M. Long-term
variability in serum thyroglobulin and thyroid related hormones in healthy
subjects. Acta Endocrinol (Copenh)1980;95:328-34.
- Browning MCK, Ford RP, Callaghan SJ and Fraser CG. Intra-and interindividual
biological variation of five analytes used in assessing thyroid function:
implications for necessary standards of performance and the interpretation of
results. Clin Chem 1986;32:962-6.
- Lum SM and Nicoloff JT. Peripheral tissue mechanism for maintenance of serum
triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest
1984;73:570-5.
- Spencer CA and Wang CC. Thyroglobulin measurement:- Techniques, clinical
benefits and pitfalls. Endocrinol Metab Clin N Amer 1995;24:841-63.
- Weeke J and Gundersen HJ. Circadian and 30 minute variations in serum TSH
and thyroid hormones in normal subjects. Acta Endocrinol 1978;89:659-72.
- Brabant G, Prank K, Hoang-Vu C and von zur Muhlen A. Hypothalamic regulation
of pulsatile thyrotropin secretion. J Clin Endocrinol Metab 1991;72:145-50.
- Fraser CG, Petersen PH, Ricos C and Haeckel R. Proposed quality
specifications for the imprecision and inaccuracy of analytical systems for
clinical chemistry. Eur J Clin Chem Biochem 1992;30:311-7.
- Rodbard, D. Statistical estimation of the minimal detectable concentration
("sensitivity") for radioligand assays. Anal Biochem 1978;90:1-12.
- Ekins R and Edwards P. On the meaning of "sensitivity". Clin Chem
1997;43:1824-31.
- Fuentes-Arderiu X and Fraser CG. Analytical goals for interference. Ann Clin
Biochem 1991;28:393-5.
- Petersen PH, Fraser CG, Westgard JO and Larsen ML. Analytical goal-setting
for monitoring patients when two analytical methods are used. Clin Chem
1992;38:2256-60.
- Fraser CG and Petersen PH. Desirable standards for laboratory tests if they
are to fulfill medical needs. Clin Chem 1993;39:1453-5.
- Stockl D, Baadenhuijsen H, Fraser CG, Libeer JC, Petersen PH and Ricos C.
Desirable routine analytical goals for quantities assayed in serum. Discussion
paper from the members of the external quality assessment (EQA) Working Group A
on analytical goals in laboratory medicine. Eur J Clin Chem Clin Biochem
1995;33:157-69.
- Plebani M, Giacomini A, Beghi L, de Paoli M, Roveroni G, Galeotti F, Corsini
A and Fraser CG. Serum tumor markers in monitoring patients: interpretation of
results using analytical and biological variation. Anticancer Res
1996;16:2249-52.
- Browning MC, Bennet WM, Kirkaldy AJ and Jung RT. Intra-individual variation
of thyroxin,triiodothyronine and thyrotropin in treated hypothyroid patients:
implications for monitoring replacement therapy. Clin Chem 1988;34:696-9.
- Harris EK. Statistical principles underlying analytic goal-setting in
clinical chemistry. Am J Clin Pathol 1979;72:374-82.
- Nelson JC and Wilcox RB. Analytical performance of free and total thyroxine
assays. Clin Chem1996;42:146-54.
- Evans SE, Burr WA and Hogan TC. A reassessment of 8-anilino-1-napthalene
sulphonic acid as a thyroxine binding inhibitor in the radioimmunoassay of
thyroxine. Ann Clin Biochem 1977;14:330-4.
- Karapitta CD, Sotiroudis TG, Papadimitriou A and Xenakis A. Homogeneous
enzyme immunoassay for triiodothyronine in serum. Clin Chem 2001;47:569-74.
- De Brabandere VI, Hou P, Stockl D, Theinpont LM and De Leenheer AP. Isotope
dilution-liquid chromatography/electrospray ionization-tandem mass spectrometry
for the determination of serum thyroxine as a potential reference method. Rapid
Commun Mass Spectrom 1998;12:1099-103.
- Tai SSC, Sniegoski LT and Welch MJ. Candidate reference method for total
thyroxine in human serum: Use of isotope-dilution liquid chromatography-mass
spectrometry with electrospray ionization. Clin Chem 2002;48:637-42.
- Thienpont LM, Fierens C, De Leenheer AP and Przywara L. Isotope dilution-gas
chromatography/mass spectrometry and liquid chromatography/electro-spray
ionization-tandem mass spectrometry for the determination of triiodo-L-thyronine
in serum. Rapid Commun Mass Spectrom1999;13:1924-31.
- Sarne DH, Refetoff S, Nelson JC and Linarelli LG. A new inherited
abnormality of thyroxine-binding globulin (TBG-San Diego) with decreased
affinity for thyroxine and triiodothyronine. J Clin Endocrinol Metab
1989;68:114-9.
- Schussler GC. The thyroxine-binding proteins. Thyroid 2000;10:141-9.
- Beck-Peccoz P, Romelli PB, Cattaneo MG, Faglia G, White EL, Barlow JW et al.
Evaluation of free T4 methods in the presence of iodothyronine autoantibodies. J
Clin Endocrinol Metab 1984;58:736-9.
- Sakata S, Nakamura S and Miura K. Autoantibodies against thyroid hormones or
iodothyronine. Ann Intern Med 1985;103:579-89.
- Despres N and Grant AM. Antibody interference in thyroid assays: a potential
for clinical misinformation. Clin Chem 1998;44:440-54.
- Hay ID, Bayer MF, Kaplan MM, Klee GG, Larsen PR and Spencer CA. American
Thyroid Association Assessment of Current Free Thyroid Hormone and Thyrotropin
Measurements and Guidelines for Future Clinical Assays. Clin Chem 1991;37:2002 -
2008.
- Ekins R. The science of free hormone measurement. Proc UK NEQAS Meeting
1998;3:35-59.
- Wang R, Nelson JC, Weiss RM and Wilcox RB. Accuracy of free thyroxine
measurements across natural ranges of thyroxine binding to serum proteins.
Thyroid 2000;10:31-9.
- Nelson JC, Wilcox BR and Pandian MR. Dependence of free thyroxine estimates
obtained with equilibrium tracer dialysis on the concentration of
thyroxine-binding globulin. Clin Chem1992;38:1294-1300.
- Ekins R. The free hormone hypothesis and measurement of free hormones. Clin
Chem 1992;38:1289-93.
- Ekins RP. Ligand assays: from electrophoresis to miniaturized microarrays.
Clin Chem 1998;44:2015-30.
- Ekins R. Analytic measurements of free thyroxine. Clin Lab Med
1993;13:599-630.
- Nusynowitz, M. L. Free-thyroxine index. JAMA 1975;232:1050.
- Larsen PR, Alexander NM, Chopra IJ, Hay ID, Hershman JM, Kaplan MM et al.
Revised nomenclature for tests of thyroid hormones and thyroid-related proteins
in serum. J Clin Endocrinol Metab 1987;64:1089-94.
- Burr WA, Evans SE, Lee J, Prince HP, Ramsden DB. The ratio of thyroxine to
thyroxine-binding globulin measurement in the evaluation of thyroid function.
Clin Endocrinol 1979;11:333-42.
- Attwood EC and Atkin GE. The T4: TBG ratio: a re-evaluation with particular
reference to low and high serum TBG levels. Ann Clin Biochem 1982;19:101-3.
- Szpunar WE, Stoffer SS and DiGiulio W. Clinical evaluation of a thyroxine
binding globulin assay in calculationg a free thyroxine index in normal, thyroid
disease and sick euthyroid patients. J Nucl Med1987;28:1341-3.
- Nelson JC and Tomei RT. Dependence of the thyroxin/thyroxin-binding globulin
(TBG) ratio and the free thyroxin index on TBG concentrations. Clin Chem
1989;35:541-4.
- Sterling K and Brenner MA. Free thyroxine in human serum: Simplified
measurement with the aid of magnesium precipitation. J Clin Invest
1966;45:153-60.
- Schulssler GC and Plager JE. Effect of preliminary purification of
131-Thyroxine on the determination of free thyroxine in serum. J Clin Endocrinol
1967;27:242-50.
- Nelson JC and Tomei RT. A direct equilibrium dialysis/radioimmunoassay
method for the measurement of free thyroxin in undiluted serum. Clin Chem
1988;34:1737-44.
- Tikanoja SH. Ultrafiltration devices tested for use in a free thyroxine
assay validated by comparison with equilibrium dialysis. Scand J Clin Lab Invest
1990;50:663-9.
- Ellis SM and Ekins R. Direct measurement by radioimmunoassay of the free
thyroid hormone concentrations in serum. Acta Endocrinol (Suppl)
1973;177:106-110.
- Weeke J and Orskov H. Ultrasensitive radioimmunoassay for direct
determination of free triiodothyronine concentration in serum. Scand J Clin Lab
Invest 1975;35:237-44.
- Surks MI, Hupart KH, Chao P and Shapiro LE. Normal free thyroxine in
critical nonthyroidal illnessess measured by ultrafiltration of undiluted serum
and equilibrium dialysis. J Clin Endocrinol Metab 1988;67:1031-9.
- Holm SS andreasen L, Hansen SH, Faber J and Staun-Olsen P. Influence of
adsorption and deproteination on potential free thyroxine reference methods.
Clin Chem 2002;48:108-114.
- Jaume JC, Mendel CM, Frost PH,Greenspan FS, Laughton CW. Extremely low doses
of heparin release lipase activity into the plasma and can thereby cause
artifactual elevations in the serum-free thyroxine concentrations as measured by
equilibrium dialysis. Thyroid 1996;6:79-83.
- Stevenson HP, Archbold GP, Johnston P, Young IS, Sheridan B. Misleading
serum free thyroxine results during low molecular weight heparin treatment. Clin
Chem 1998;44:1002-7.
- Laji K, Rhidha B, John R, Lazarus J and Davies JS. Artifactual elevations in
serum free thyroxine and triiodothyronine concentrations during heparin therapy.
QJM 2001;94:471-3.
- Lim CF, Bai Y, Topliss DJ, Barlow JW and Stockigt JR. Drug and fatty acid
effects on serum thyroid hormone binding. J Clin Endocrinol Metab 1988;67:682-8.
- Czako, G., M. H. Zweig, C. Benson and M. Ruddel. On the albumin-dependence
of measurements of free thyroxin. II Patients with non-thyroidal illness. Clin
Chem 1987;33:87-92.
- Csako G, Zwieg MH, Glickman J, Ruddel M and K. J. Direct and indirect
techniques for free thyroxin compared in patients with nonthyroidal illness. II.
Effect of prealbumin, albumin and thyroxin-binding globulin. Clin Chem
1989;35:1655-62.
- Csako G, Zweig MH, Glickman J, Kestner J and Ruddel M. Direct and indirect
techniques for free thyroxin compared in patients with nonthyroidal illness. I.
Effect of free fatty acids. Clin Chem1989;35:102-9.
- Ross HA and Benraad TJ. Is free thyroxine accurately measurable at room
temperature? Clin Chem1992;38:880-6.
- Van der Sluijs Veer G, Vermes I, Bonte HA and Hoorn RKJ. Temperature effects
on Free Thyroxine Measurement: Analytical and Clinical Consequences. Clin Chem
1992;38:1327-31.
- Fisher DA. The hypothyroxinemia of prematurity. J Clin Endocrinol Metab
1997;82:1701-3.
- Stockigt JR, Stevens V, White EL and Barlow JW. Unbound analog
radioimmunoassays for free thyroxin measure the albumin-bound hormone fraction.
Clin Chem 1983;29:1408-10.
- Aravelo G. Prevalence of familial dysalbuminemic hyperthyroxinemia in serum
samples received for thyroid testing. Clin Chem 1991;37:1430-1.
- Sapin R and Gasser F. Anti-solid phase antibodies interfering in
labeled-antibody assays for free thyroid hormones. Clin Chem 1995;45:1790-1.
- Inada M and Sterling K. Thyroxine transport in thyrotoxicosis and
hypothyroidism. J Clin Invest1967;46:1442-50.
- Lueprasitsakul W, Alex S, Fang SL, Pino S, Irmscher K, Kohrle J et al.
Flavonoid administration immediately displaces thyroxine (T4) from serum
transthyretin, increases serum free T4 and decreases serum thyrotropin in the
rat. Endocrinol 1990;126:2890-5.
- Stockigt JR, Lim CF, Barlow J, Stevens V, Topliss DJ, Wynne KN. High
concentrations of furosemide inhibit plasma binding of thyroxine. J Clin
Endocrinol Metab 1984;59:62-6.
- Hawkins RC. Furosemide interference in newer free thyroxine assays. Clin
Chem 1998;44:2550-1.
- Wang R, Nelson JC and Wilcox RB. Salsalate and salicylate binding to and
their displacement of thyroxine from thyroxine-binding globulin, transthyrin and
albumin. Thyroid 1999;9:359-64.
- Munro SL, Lim C-F, Hall JG, Barlow JW, Craik DJ, Topliss DJ and Stockigt JR.
Drug competition for thyroxine binding to transthyretin (prealbumin): comparison
with effects on thyroxine-binding globulin. J Clin Endocrinol Metab
1989;68:1141-7.
- Stockigt JR, Lim C-F, Barlow JW and Topliss DJ. 1997. Thyroid hormone
transport. Springer Verlag,Heidelberg. 119 pp.
- Surks MI and Defesi CR. Normal free thyroxine concentrations in patients
treated with phenytoin or carbamazepine: a paradox resolved. JAMA
1996;275:1495-8.
- Ross HA. A dialysis method for the measurement of free iodothyronine and
steroid hormones in blood. Experientia 1978;34:538-9.
- Sapin R. Serum thyroxine binding capacity-dependent bias in five free
thyroxine immunoassays:assessment with serum dilution experiments and impact on
diagnostic performance. Clin Biochem2001;34:367-71.
- Law LK, Cheung CK and Swaminathan R. Falsely high thyroxine results by
fluorescence polarization in sera with high background fluorescence. Clin Chem
1988;34:1918.
- Kricka LJ. Interferences in Immunoassay - still a threat. Clin Chem
2000;46:1037-8.
- McBride JH, Rodgerson DO and Allin RE. Choriogonadotrophin interference in a
sensitive assay for Thyrotropin. Clin Chem 1987;33:1303-4.
- Ritter D, Stott R, Grant N and Nahm MH. Endogenous antibodies that interfere
with Thyroxine fluorescence polarization assay but not with radioimmunoassay or
EMIT. Clin Chem 1993;39:508-11.
- DeGroot LJ, Larsen PR, Refetoff S and Stanbury JB. The Thyroid and its
Diseases. Fifth Edition,1984;John Wiley & Sons, Inc., New York:266-7.
- Beck-Peccoz P, Amr S, Menezes-Ferreira NM, Faglia G and Weintraub BD.
Decreased receptor binding of biologically inactive thyrotropin in central
hypothyroidism: effect of treatment with thyrotropin-releasing hormone. N Engl J
Med 1985;312:1085-90.
- Beck-Peccoz P and Persani L. Variable biological activity of
thyroid-stimulating hormone. Eur J Endocrinol 1994;131:331-40.
- Persani L, Ferretti E, Borgato S, Faglia G and Beck-Peccoz P. Circulating
thyrotropin bioactivity in sporadic central hypothyroidism. J Clin Endocrinol
Metab 2000;85:3631-5.
- Rafferty B and Gaines Das R. Comparison of pituitary and recombinant humaN
thyroid-stimulating hormone (rhTSH) in a multicenter collaborative study:
establishment of the first World Health Organization reference reagent for
rhTSH. Clin Chem 1999;45:2207-15.
- Persani L, Borgato S, Romoli R, Asteria C, Pizzocaro A and Beck-Peccoz P.
Changes in the degree of sialylation of carbohydrate chains modify the
biological properties of circulating thyrotropin isoforms in various
physiological and pathological states. J Clin Endocrinol Metab 1998;83:2486-92.
- Gershengorn MC and Weintraub BD. Thyrotropin-induced hyperthyroidism caused
by selective pituitary resistance to thyroid hormone. A new syndrome of
"inappropriate secretion of TSH". J Clin Invest 1975;56:633-42.
- Faglia G, Beck-Peccoz P, Piscitelli G and Medri G. Inappropriate secretion
of thyrotropin by the pituitary. Horm Res 1987;26:79-99.
- Spencer CA, Takeuchi M and Kazarosyan M. Current status and performance
goals for serum thyrotropin (TSH) assays. Clinical Chemistry 1996;42:141-145.
- Laurberg P. Persistent problems with the specificity of immunometric TSH
assays. Thyroid1993;3:279-83.
- Spencer CA, Schwarzbein D, Guttler RB, LoPresti JS and Nicoloff JT. TRH
stimulation test responses employing third and fourth generation TSH assays. J
Clin Endocrinol Metab 1993;76:494-498.
- Vogeser M, Weigand M, Fraunberger P, Fischer H and Cremer P. Evaluation of
the ADVIA Centaur TSH-3 assay. Clin Chem Lab Med 2000;38:331-4.
- Spencer CA, Takeuchi M, Kazarosyn M, MacKenzie F, Beckett GJ and Wilkinson
E. Interlaboratory/intermethod differences in functional sensitivity of
immunometric assays for hyrotropin (TSH): impact on reliability of measurement
of subnormal concentration. Clin Chem1995;41:367-74.
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG,
Young E, Bird T and Smith PA. The spectrum of thyroid disease in a community:
the Whickham survey. Clin Endocrinol1977;7:481-93.
- Rago T, Chiovato L, Grasso L, Pinchera A and Vitti P. Thyroid
ultrasonography as a tool for detecting thyroid autoimmune diseases and
predicting thyroid dysfunction in apparently healthy subjects. J Endocrinol
Invest 2001;24:763-9.
- Hershman JM and Pittman JA. Utility of the radioimmunoassay of serum
thyrotropin in man. Ann Intern Med 1971;74:481-90.
- Becker DV, Bigos ST, Gaitan E, Morris JC, Rallison ML, Spencer CA, Sugawara
M, Middlesworth LV and Wartofsky L. Optimal use of blood tests for assessment of
thyroid function. JAMA1993;269:2736.
- Canaris GJ, Manowitz NR, Mayor G and Ridgway EC. The Colorado Thyroid
Disease Prevalence Study. Arch Intern Med 2000;160:19-27.
- Skamene A and Patel YC. Infusion of graded concentrations of somatostatin in
man: pharmacokinetic and differential inhibitory effects on pituitary and islet
hormones. Clin Endocrinol 1984;20:555-64.
- Berghout A, Wiersinga WM, Smits NJ and Touber JL. Interrelationships between
age, thyroid volume,thyroid nodularity and thyroid function in patients with
sporadic nontoxic goiter. Am J Med1990;89:602-8.
- Parle JV, Franklyn JA, Cross KW, Jones SC and Sheppard MC. Prevalence and
follow-up of abnormal thyrotropin (TSH) concentrations in the elderly in the
United Kingdom. Clin Endocrinol 1991;34:77-83.
- Danese D, Sciacchitano S, Farsetti A andreoli M and Pontecorvi A. Diagnostic
accuracy of conventional versus sonography-guided fine-needle aspiration biopsy
of thyroid nodules. Thyroid1998;8:15-21.
- McDermott MT and Ridgway EC. Subclinical hypothyroidism is mild thyroid
failure and should be treated. J Clin Endocrinol Metab 2001;86:4585-90.
- Chu JW and Crapo LM. The treatment of subclinical hypothyroidism is seldom
necessary. J Clin Endocrinol Metab 2001;86:4591-9.
- Lewis GF, Alessi CA, Imperial JG and Refetoff S. Low serum free thyroxine
index in ambulating elderly is due to a resetting of the threshold of
thyrotropin feedback suppression. JCEM 1991;73:843-9.
- Pearce CJ and Himsworth RL. Total and free thyroid hormone concentrations in
patients receiving maintenance replacement treatment with thyroxine. Br Med J
1984;288:693-5.
- Fish LH, Schwarz HL, Cavanaugh MD, Steffes MW, Bantle JP, Oppenheimer JH.
Replacement dose,metabolism and bioavailability of levothyroxine in the
treatment of hypothyroidism. N Engl J Med1987;316:764-70.
- Sawin CT, Herman T, Molitch ME, London MH and Kramer SM. Aging and the
thyroid. Decreased requirement for thyroid hormone in older hypothyroid
patients. Amer J Med 1983;75:206-9.
- Davis FB, LaMantia RS, Spaulding SW, Wemann RE and Davis PJ. Estimation of a
physiologic replacement dose of levothyroxine in elderly patients with
hypothyroidism. Arch Intern Med 1984;144.
- Arafah BM. Estrogen therapy may necessitate an increase in thyroxine dose
for hypothyroidism. NEJM 2001;344:1743-9.
- Scheithauer BW, Kovacs K, Randall RV and Ryan N. Pituitary gland in
hypothyroidism. Histologic and immunocytologic study. Arch Pathol Lab Med
1985;109:499-504.
- Ain KB, Pucino F, Shiver T and Banks SM. Thyroid hormone levels affected by
time of blood sampling in thyroxine-treated patients. Thyroid 1993;3:81-5.
- Chorazy PA, Himelhoch S, Hopwood NJ, Greger NG and Postellon DC. Persistent
hypothyroidism in an infant receiving a soy formula: case report and review of
the literature. Pediatrics 1995;96:148-50.
- Dulgeroff AJ and Hershman JM. Medical therapy for differentiated thyroid
carcinoma. Endocrinol Rev1994;15:500-15.
- Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J and Jaffiol C. Degree of
thyrotropin suppression as a prognostic determinant in differentiated thyroid
cancer. J Clin Endocrinol Metab 1996;81:4318-23.
- Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, Ain KB, Bigos
ST, Brierley JD,Haugen BR, Klein I, Robbins J, Sherman SI, Taylor T and Maxon HR
3rd. Thyrotropin suppression and disease progression in patients with
differentiated thyroid cancer: results from the National thyroid Cancer
Treatment Cooperative Registry. Thyroid 1999;8:737-44.
- Hurley DL and Gharib H. Evaluation and management of multinodular goiter.
Otolaryngol Clin North Am 1996;29:527-40.
- Bayer MF, Macoviak JA and McDougall IR. Diagnostic performance of sensitive
measurements of serum thyrotropin during severe nonthyroidal illness: Their role
in the diagnosis of hyperthyroidism. Clin Chem 1987;33:2178-84.
- Lum SM, Kaptein EM and Nicoloff JT. Influence of nonthyroidal illnesses on
serum thyroid hormone indices in hyperthyroidism. West J Med 1983;138:670-5.
- Faglia G, Bitensky L, Pinchera A, Ferrari C, Paracchi A, Beck-Peccoz P,
Ambrosi B and Spada A. Thyrotropin secretion in patient with central
hypothyroidism: Evidence for reduced biological activity of immunoreactive
thyrotropin. J Clin Endocrinol Metab 1979;48:989-98.
- Faglia G, Beck-Peccoz P, Ballabio M and Nava C. Excess of beta-subunit of
thyrotropin (TSH) in patients with idiopathic central hypothyroidism due to the
secretion of TSH with reduced biological activity. J Clin Endocrinol Metab
1983;56:908-14.
- Faglia G. The clinical impact of the thyrotropin-releasing hormone test.
Thyroid 1998;8:903-8.
- Trejbal D, Sulla I, Trejbalova L, Lazurova I, Schwartz P and Machanova Y.
Central hypothyroidism -various types of TSH responses to TRH stimulation.
Endocr Regul 1994;28:35-40.
- Faglia G, Ferrari C, Paracchi A, Spada A and Beck-Peccoz P. Triiodothyronine
response to thyrotropin releasing hormone in patients with
hypothalamic-pituitary disorders. Clin Endocrinol 1975;4:585-90.
- Horimoto M, Nishikawa M, Ishihara T, Yoshikawa N, Yoshimura M and Inada M.
Bioactivity of thyrotropin (TSH) in patients with central hypothyroidism:
comparison between in vivo 3,5,3'-triiodothyronine response to TSH and in vitro
bioactivity of TSH. J Clin Endocrinol Metab1995;80:1124-8.
- Refetoff S, Weiss RE and Usala SJ. The syndromes of resistance to thyroid
hormone. Endocr Rev1993;14:348-99.
- Weiss RE, Hayashi Y, Nagaya T, Petty KJ, Murata Y, Tunca H, Seo H and
Refetoff S. Dominant inheritance of resistance to thyroid hormone not linked to
defects in the thyroid hormone receptors alpha or beta genes may be due to a
defective co-factor. J Clin Endocrinol Metab 1996;81:4196-203.
- Snyder D, Sesser D, Skeels M et al. Thyroid disorders in newborn infants
with elevated screening T4. Thyroid 1997;7 (Suppl 1):S1-29 (abst).
- Refetoff S. 2000. Resistance to Thyroid Hormone. In The Thyroid. Braverman
LE and Utiger RD,editor. Lippincott Williams & Wilkins, Philadelphia.
1028-43.
- Beck-Peccoz P and Chatterjee VKK. The variable clinical phenotype in thyroid
hormone resistance syndrome. Thyroid 1994;4:225-32.
- Persani L, Asteria C, Tonacchera M, Vitti P, Krishna V, Chatterjee K and
Beck-Peccoz P. Evidence for the secretion of thyrotropin with enhanced
bioactivity in syndromes of thyroid hormone resistance. J Clin Endocrinol Metab
1994;78:1034-9.
- Sarne DH, Sobieszczyk S, Ain KB and Refetoff S. Serum thyrotropin and
prolactin in the syndrome of generalized resistance to thyroid hormone:
responses to thyrotrophin-releasing hormone stimulation and triiodothyronine
suppression. J Clin Endocrinol Metab 1990;70:1305-11.
- Ercan-Fang S, Schwartz HL, Mariash CN and Oppenheimer JH. Quantitative
assessment of pituitary resistance to thyroid hormone from plots of the
logarithm of thyrotropin versus serum free thyroxine index. J Clin Endocrinol
Metab 2000;85:2299-303.
- Safer JD, Colan SD, Fraser LM and Wondisford FE. A pituitary tumor in a
patient with thyroid hormone resistance: a diagnostic dilemma. Thyroid
2001;11:281-91.
- Marcocci C and Chiovato L. 2000. Thyroid -directed antibodies. In Thyroid.
B. L. a. U. RD, editor. Lippincott Williams and Wilkins, Philadelphia. 414-31.
- Chiovato L, Bassi P, Santini F, Mammoli C, Lapi P, Carayon P and Pinchera A.
Antibodies producing complement-mediated thyroid cytotoxicity in patients with
atrophic or goitrous autoimmune thyroiditis. J Clin Endocrinol Metab
1993;77:1700-5.
- Guo J, Jaume JC, Rapoport B and McLachlan SM. Recombinant thyroid
peroxidase-specific Fab converted to immunoglobulin G (IgG)molecules: evidence
for thyroid cell damage by IgG1, but not IgG4, autoantibodies. J Clin Endocrinol
Metab 1997;82:925-31.
- Doullay F, Ruf J, Codaccioni JL and Carayon P. Prevalence of autoantibodies
to thyroperoxidase in patients with various thyroid and autoimmune diseases.
Autoimmunity 1991;9:237-44.
- Radetti G, Persani L, Moroder , Cortelazzi D, Gentili L, Beck-Peccoz P.
Transplacental passage of anti-thyroid autoantibodies in a pregnant woman with
auto-immune thyroid disease. Prenatal Diagnosis1999;19:468-71.
- Heithorn R, Hauffa BP and Reinwein D. Thyroid antibodies in children of
mothers with autoimmune thyroid disorders. Eur J Pediatr 1999;158:24-8.
- Feldt-Rasmussen. Anti-thyroid peroxidase antibodies in thyroid disorders and
non thyroid autoimmune diseases. Autoimmunity 1991;9:245-51.
- Mariotti S, Chiovato L, Franceschi C and Pinchera A. Thyroid autoimmunity
and aging. Exp Gerontol1999;33:535-41.
- Ericsson UB, Christensen SB and Thorell JI. A high prevalence of
thyroglobulin autoantibodies in adults with and without thyroid disease as
measured with a sensitive solid-phase immunosorbent radioassay. Clin Immunol
Immunopathol 1985;37:154-62.
- Feldt-Rasmussen U, Hoier-Madsen M, Rasmussen NG, Hegedus L and Hornnes P.
Anti-thyroid peroxidase antibodies during pregnancy and postpartum. Relation to
postpartum thyroiditis. Autoimmunity 1990;6:211-4.
- Premawardhana LD, Parkes AB, AMMARI F, John R, Darke C, Adams H and Lazarus
JH. Postpartum thyroiditis and long-term thyroid status: prognostic influence of
Thyroid Peroxidase Antibodies and ultrasound echogenicity. J Clin Endocrinol
Metab 2000;85:71-5.
- Johnston AM and Eagles JM. Lithium-associated clinical hypothyroidism.
Prevalence and risk factors. Br. J Psychiatry 1999;175:336-9.
- Bell TM, Bansal AS, Shorthouse C, Sandford N and Powell EE. Low titre
autoantibodies predict autoimmune disease during interferon alpha treatment of
chronic hepatitis C. J Gastroenterol Hepatol1999;14:419-22.
- Ward DL and Bing-You RG. Autoimmune thyroid dysfunction induced by
interfereon-alfa treatment for chronic hepatitis C: screening and monitoring
recommendations. Endoc Pract 2001;7:52-8.
- Carella C, Mazziotti G, Morisco F, Manganella G, Rotondi M, Tuccillo C,
Sorvillo F, Caporaso N and Amato G. Long-term outcome of
interferon-alpha-induced thyroid autoimmunity and prognostic influence of
thyroid autoantibody pattern at the end of treatment. J Clin Endocrinol
Metab2001;86:1925-9.
- Feldt-Rasmussen U, Schleusener H and Carayon P. Meta-analysis evaluation of
the impact of thyrotropin receptor antibodies on long term remission after
medical therapy of Graves' disease. J Clin Endocrinol Metab 1994;78:98-103.
- Estienne V, Duthoit C, Di Costanzo, Lejeune PJ, Rotondi M, Kornfeld S et al.
Multicenter study on TGPO autoantibodies prevalence in various thyroid and
non-thyroid diseases: relationships with thyroglobulin and thyroperoxidase
autoantibody parameters. Eur J Endocrinol 1999;141:563-9.
- Czarnocka B, Ruf J, Ferrand M et al. Purification of the human thyroid
peroxidase and its identification as the microsomal antigen involved in
autoimmune thyroid diseases. FEBS Lett1985;190:147-52.
- Mariotti S, Caturegli P, Piccolo P, Barbesino G and Pinchera A. Antithyroid
peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab
1990;71:661-9.
- Rubello D, Pozzan GB, Casara D, Girelli ME, Boccato s, Rigon F, Baccichetti
C, Piccolo M, Betterle C and Busnardo B. Natural course of subclinical
hypothyroidism in Down's syndrome: prospective study results and therapeutic
considerations. J Endocrinol Invest 1995;18:35-40.
- Karlsson B, Gustafsson J, Hedov G, Ivarsson SA and Anneren G. Thyroid
dysfunction in Down's syndrome: relation to age and thyroid autoimmunity. Arch
Dis Child 1998;79:242-5.
- Bussen S, Steck T and Dietl J. Increased prevalence of thyroid antibodies in
euthyroid women with a history of recurrent in-vitro fertilization failure. Hum
Reprod 2000;15:545-8.
- Phan GQ, Attia P, Steinberg SM, White DE and Rosenberg SA. Factors
associated with response to high-dose interleukin-2 in patients with metastatic
melanoma. J Clin Oncol 2001;19:3477-82.
- Durelli L, Ferrero B, Oggero A, Verdun E, Ghezzi A, Montanari E and
Zaffaroni M. Thyroid function and autoimmunity during interferon-Beta-1b
Treatment: a Multicenter Prospective Study. J Clin Endocrinol Metab
2001;86:3525-32.
- Roti E, Minelli R, Giuberti T, Marchelli C, Schianchi C, Gardini E, Salvi M,
Fiaccadori F, Ugolotti G,Neri TM and Braverman LE. Multiple changes in thyroid
function in patients with chronic active HCV hepatitis treated with recombinant
interferon-alpha. Am J Med 1996;101:482-7.
- Ruf J, Carayon P and Lissitzky S. Various expression of a unique anti-human
thyroglobulin antibody repertoire in normal state and autoimmune disease. Eur J
Immunol 1985;15:268-72.
- Ruf J, Toubert ME, Czarnocka B, Durand-Gorde JM,Ferrand M, Carayon P.
Relationship between immunological structure and biochemical properties of human
thyroid peroxidase. Endocrinol1989;125:1211-8.
- Feldt-Rasmussen U and Rasmussen A K. Serum thyroglobulin (Tg)in presence of
thyroglobulin autoantibodies (TgAb). Clinical and methodological relevance of
the interaction between Tg and TgAb in vivo and in vitro. J Endocrinol Invest
1985;8:571-6.
- Spencer CA, Wang C, Fatemi S, Guttler RB, Takeuchi M and Kazarosyan M. Serum
Thyroglobulin Autoantibodies: Prevalence, influence on serum thyroglobulin
measurement and prognostic significance in patients with differentiated thyroid
carcinoma. J Clin Endocrinol Metab 1998;83:1121-7.
- Pacini F, Mariotti S, Formica N and Elisei R. Thyroid autoantibodies in
thyroid cancer: Incidence and relationship with tumor outcome. Acta Endocrinol
1988;119:373-80.
- Rubello D, Casara D, Girelli ME, Piccolo M and Busnardo B. Clinical meaning
of circulating antithyroglobulin antibodies in differentiated thyroid cancer: a
prospective study. J Nucl Med1992;33:1478-80.
- Nordyke RA, Gilbert FI, Miyamoto LA and Fleury KA. The superiority of
antimicrosomal over antithyroglobulin antibodies for detecting Hashimoto's
thyroiditis. Arch Intern Med 1993;153:862-5.
- Di Cerbo A, Di Paoloa R, Menzaghi C, De Filippis V, Tahara K, Corda D et al.
Graves'immunoglobulins activate phospholipase A2 by recognizing specific
epitopes on the thyrotropin receptor. J Clin Endocrinol Metab 1999;84:3283-92.
- Kung AWC, Lau KS and Kohn LD. Epitope mapping of TSH Receptor-blocking
antibodies in Graves'disease that appear during pregnancy. J Clin Endocrinol
Metab 2001;86:3647-53.
- Ueta Y, Fukui H, Murakami M, Yamanouchi Y, Yamamoto R, Murao A et al.
Development of primary hypothyroidism with the appearance of blocking-type
antibody to thyrotropin receptor in Graves'disease in late pregnancy. Thyroid
1999;9:179-82.
- Gupta MK. Thyrotropin-receptor antibodies in thyroid diseases: advances in
detection techniques and clinical application. Clin Chem Acta 2000;293:1-29.
- Kung AW, Lau KS and Kohn LD. Characterization of thyroid-stimulating
blocking antibodies that appeared during transient hypothyroidism after
radioactive iodine therapy. Thyroid 2000;10:909-17.
- Filetti S, Foti D, Costante G and Rapoport B. Recombinant human thyrotropin
(TSH) receptor in a radioreceptor assay for the measurement of TSH receptor
antibodies. J Clin Endocrinol Metab1991;72:1096-101.
- Adams DD and Purves HD. Abnormal responses in the assay of thyrotropin. Proc
Univ Otago Med Sch 1956;34:11-12.
- Morgenthaler NG. New assay systems for thyrotropin receptor antibodies.
Current Opinion Endocrinol Diabetes 1998;6:251-60.
- Kamijo K, Nagata A and Sato Y. Clinical significance of a sensitive assay
for thyroid-stimulating antibodies in Graves' disease using polyethylene glycol
at high concentration and porcine thyroid cells. Endocrinol J 1999;46:397-403.
- Takasu N, Yamashiro K, Ochi Y, Sato Y, Nagata A, Komiya I et al. TSBAb
(TSH-Stimulation Blocking Antibody) and TSAb (Thyroid Stimulating Antibody) in
TSBAb-positive patients with hypothyroidism and Graves' patients with
hyperthyroidism. Horm Metab Res 2001;33:232-7.
- Costagliola S, Swillens S, Niccoli P, Dumont JE, Vassart G and Ludgate M.
Binding assay for thyrotropin receptor autoantibodies using the recombinant
receptor protein. J Clin Endocrinol Metab1992;75:1540-44.
- Morgenthaler NG, Hodak K, Seissler J, Steinbrenner H, Pampel I, Gupta M et
al. Direct binding of thyrotropin receptor autoantibody to in vitro translated
thyrotropin receptor: a comparison to radioreceptor assay and thyroid
stimulating bioassay. Thyroid 1999;9:466-75.
- Akamizu T, Inoue D, Kosugi S, Kohn LD and Mori T. Further studies of amino
acids (268-304) in thyrotropin (TSH)-lutropin/chorionic gonadotropin (LH/CG)
receptor chimeras: Cysteine-301 is important in TSH binding and receptor
tertiary structure. Thyroid 1994;4:43-8.
- Grasso YZ, Kim MR, Faiman C, Kohn LD, Tahara K and Gupta MK. Epitope
heterogeneity of thyrotropin-blocking antibodies in Graves' patients as detected
with wild-type versus chimeric thyrotropin receptors. Thyroid 1999;9:521-37.
- Kim WB, Chung HK, Lee HK, Kohn LD, Tahara K and Cho BY. Changes in epitopes
for thyroid stimulation antibodies in Graves' disease sera during treatment of
hyperthyroidism: Therapeutic implications. J Clin Endocrinol Metab
1997;82:1953-9.
- Shewring G and Smith BR. An improved radioreceptor assay for TSH receptor.
Methods Enzymol1982;17:409-17.
- Costagliola S, Morganthaler NG, Hoermann R, Badenhoop K, Struck J, Freitag
D, Poertl S, Weglohner W, Hollidt JM, Quadbeck B, Dumont JE, Schumm-Draeger PM,
Bergmann A, Mann K, Vassart G and Usadel KH. Second generation assay for
thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves'
disease. J Clin Endocrinol Metab 1999;84:90-7.
- Schott M, Feldkamp J, Bathan C, Fritzen R, Scherbaum WA and Seissler J.
Detecting TSH-Receptor antibodies with the recombinant TBII assay: Technical and
Clinical evaluation. 32 2000;:429-35.
- Feldt-Rasmussen U. Analytical and clinical performance goals for testing
autoantibodies to thyroperoxidase, thyroglobulin and thyrotropin receptor. Clin
Chem 1996;42:160-3.
- Giovanella L, Ceriani L and Garancini S. Clinical applications of the 2nd.
generation assay for anti-TSH receptor antibodies in Graves' disease. Evaluation
in patients with negative 1st. generation test. Clin Chem Lab med 2001;39:25-8.
- Momotani N, Noh JY, Ishikawa N and Ito K. Effects of propylthiouracil and
methimazole on fetal thyroid status in mothers with Graves' hyperthyroidism. J
Clin Endocrinol Metab 1997;82:3633-6.
- Brown RS, Bellisario RL, Botero D, Fournier L, Abrams CA, Cower ML et al.
Incidence of transient congenital hypothyroidism due to maternal thyrotropin
receptor-blocking antibodies in over one million babies. J Clin Endocrinol Metab
1996;81:1147-51.
- Gerding MN, van der Meer Jolanda WC, Broenink M, Bakker O, W. WM and Prummel
MF. Association of thyrotropin receptor antibodies with the clinical features of
Graves' opthalmopathy. Clin Endocrinol 2000;52:267-71.
- Bartelena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell'Unto E et al.
Relation between therapy for hyperthyroidism and the course of Graves' disease.
N Engl J Med 1998;338:73-8.
- Bech K. Immunological aspects of Graves' disease and importance of thyroid
stimulating immunoglobulins. Acta Endocrinol (Copenh) Suppl 1983;103:5-38.
- Feldt-Rasmussen U. Serum thyroglobulin and thyroglobulin autoantibodies in
thyroid diseas et al.lergy1983;38:369-87.
- Nygaard B, Metcalfe RA, Phipps J, Weetman AP and Hegedus L. Graves' disease
and thyroid-associated opthalopathy triggered by 131I treatment of non-toxic
goitre. J Endocrinol Invest1999;22:481-5.
- Ericsson UB, Tegler L, Lennquist S, Christensen SB, Stahl E and Thorell JI.
Serum thyroglobulin in differentiated thyroid carcinoma. Acta Chir Scand
1984;150:367-75.
- Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI,
Cooper DS, Graham KE, Braverman LE, Skarulis MC, Davies TF, DeGroot LJ,
Mazzaferri EL, Daniels GH, Ross DS,Luster M, Samuels MH, Becker DV, Maxon HR,
Cavalieri RR, Spencer CA, McEllin K, Weintraub BD and Ridgway EC. A comparison
of recombinant human thyrotropin and thyroid hormone withdrawal for the
detection of thyroid remnant or cancer. J Clin Endocrinol Metab 1999;84:3877-85.
- Spencer CA, LoPresti JS, Fatemi S and Nicoloff JT. Detection of residual and
recurrent differentiated thyroid carcinoma by serum Thyroglobulin measurement.
Thyroid 1999;9:435-41.
- Schlumberger M, C. P., Fragu P, Lumbroso J, Parmentier C and Tubiana M.
Circulating thyrotropin and thyroid hormones in patients with metastases of
differentiated thyroid carcinoma: relationship to serum thyrotropin levels. J
Clin Endocrinol Metab 1980;51:513-9.
- Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, Elisei R
and Pinchera A. Detection of thyroglobulin in fine needle aspirates of
nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated
thyroid cancer. J Clin Endocrinol Metab 1992;74:1401-4.
- Spencer CA, Takeuchi M and Kazarosyan M. Current Status and Performance
Goals for Serum Thyroglobulin Assays. Clin Chem 1996;42:164-73.
- Feldt-Rasmussen U and Schlumberger M. European interlaboratory comparison of
serum thyroglobulin measurement. J Endocrinol Invest 1988;11:175-81.
- Feldt-Rasmussen U, Profilis C, Colinet E, Black E, Bornet H, Bourdoux P et
al. Human thyroglobulin reference material (CRM 457) 2nd part: Physicochemical
characterization and certification. Ann Biol Clin 1996;54:343-348.
- Schlumberger M J. Papillary and Follicular Thyroid Carcinoma. NEJM
1998;338:297-306.
- Hjiyiannakis P, Mundy J and Harmer C. Thyroglobulin antibodies in
differentiated thyroid cancer. Clin Oncol 1999;11:240-4.
- Spencer CA. Recoveries cannot be used to authenticate thyroglobulin (Tg)
measurements when sera contain Tg autoantibodies. Clin Chem 1996;42:661-3.
- Massart C and Maugendre D. Importance of the detection method for
thyroglobulin antibodies for the validity of thyroglobulin measurements in sera
from patients with Graves' disease. Clin Chem2002;48:102-7.
- Mariotti S, Barbesino G, Caturegli P, Marino M, Manetti L, Pacini F, Centoni
R and Pinchera A. Assay of thyroglobulin in serum with thyroglobulin
autoantibodies: an unobtainable goal? J Clin Endocrinol Metab 1995;80:468-72.
- Black EG and Hoffenberg R. Should one measure serum thyroglobulin in the
presence of anti-thyroglobulin antibodies? Clin Endocrinol 1983;19:597-601.
- Schneider AB and Pervos R. Radioimmunoassay of human thyroglobulin: effect
of antithyroglobulin autoantibodies. J Clin Endocrinol Metab 1978;47:126-37.
- Spencer CA, Platler BW and Nicoloff JT. The effect of 125-I thyroglobulin
tracer heterogeneity on serum Tg RIA measurement. Clin Chem Acta
1985;153:105-115.
- Bugalho MJ, Domingues RS, Pinto AC, Garrao A, Catarino AL, Ferreira T,
Limbert E and Sobrinho L. Detection of thyroglobulin mRNA transcripts in
peripheral blood of individuals with and without thyroid glands: evidence for
thyroglobulin expression by blood cells. Eur J Endocrinol 2001;145:409-13.
- Bellantone R, Lombardi CP, Bossola M, Ferrante A,Princi P, Boscherini M et
al. Validity of thyroglobulin mRNA assay in peripheral blood of postoperative
thyroid carcinoma patients in predicting tumor recurrence varies according to
the histologic type: results of a prospective study. Cancer 2001;92:2273-9.
- Bojunga J, Roddiger S, Stanisch M, Kusterer K, Kurek R, Renneberg H, Adams
S, Lindhorst E, Usadel KH and Schumm-Draeger PM. Molecular detection of
thyroglobulin mRNA transcripts in peripheral blood of patients with thyroid
disease by RT-PCR. Br J Cancer 2000;82:1650-5.
- Smith B, Selby P, Southgate J, Pittman K, Bradley C and Blair GE. Detection
of melanoma cells in peripheral blood by means of reverse transcriptase and
polymerase chain reaction. Lancet1991;338:1227-9.
- Luppi M, Morselli M, Bandieri E, Federico M, Marasca R, Barozzi P, Ferrari
MG, Savarino M,Frassoldati A and Torelli G. Sensitive detection of circulating
breast cancer cells by reverse-transcriptase polymerase chain reaction of maspin
gene. Ann Oncol 1996;7:619-24.
- Ghossein RA and Bhattacharya S. Molecular detection and characterisation of
circulating tumour cells and micrometastases in solid tumours. Eur J Cancer
2000;36:1681-94.
- Ditkoff BA, Marvin MR, Yemul S, Shi YJ, Chabot J, Feind C et al. Detection
of circulating thyroid cells in peripheral blood. Surgery 1996;120:959-65.
- Arturi F, Russo D, Giuffrida D et al. Early diagnosis by genetic analysis of
differentiated thyroid cancer metastases in small lymph nodes. J Clin Endocrinol
Metab 1997;82:1638-41.
- Ringel MD, Balducci-Silano PL anderson JS, Spencer CA, Silverman J, Sparling
YH, Francis GL,Burman KD, Wartofsky L, Ladenson PW, Levine MA and Tuttle RM.
Quantitative reverse transcription-polymerase chain reaction of circulating
thyroglobulin messenger ribonucleic acid for monitoring patients with thyroid
carcinoma. J Clin Endocrinol Metab 1998;84:4037-42.
- Biscolla RP, Cerutti JM and Maciel RM. Detection of recurrent thyroid cancer
by sensitive nested reverse transcription-polymerase chain reaction of
thyroglobulin and sodium/iodide symporter messenger ribonucleic acid transcripts
in peripheral blood. J Clin Endocrinol Metab 2000;85:3623-7.
- Takano T, Miyauchi A, Yoshida H, Hasegawa Y, Kuma K and Amino N.
Quantitative measurement of thyroglobulin mRNA in peripheral blood of patients
after total thyroidectomy. Br J Cancer2001;85:102-6.
- Chelly J, Concordet JP, Kaplan JC and Kahn A. Illegitimate transcription:
transcription of any gene in any cell type. Proc Natl Acad Sci USA
1989;86:2617-21.
- Premawardhana LDKE, Phillips DW, Prentice LM and Smith BR. Variability of
serum thyroglobulin levels is determined by a major gene. Clin Endocrinol
1994;41:725-9.
- Bertelsen JB and Hegedus L. Cigarette smoking and the thyroid. Thyroid
1994;4:327-31.
- Knudsen N, Bulow I, Jorgensen T, Perrild H, Oversen L and Laurberg P. Serum
Tg - a sensitive marker of thyroid abnormalities and iodine deficiency in
epidemiological studies. J Clin Endocrinol Metab 2001;86:3599-603.
- Van den Briel T, West CE, Hautvast JG, Vulsma T, de Vijlder JJ and Ategbo
EA. Serum thyroglobulin and urinary iodine concentration are the most
appropriate indicators of iodine status and thyroid function under conditions of
increasing iodine supply in schoolchildren in Benin. J Nutr2001;131:2701-6.
- Gardner DF, Rothman J and Utiger RD. Serum thyroglobulin in normal subjects
and patients with hyperthyroidism due to Graves' disease: effects of T3, iodide,
131I and antithyroid drugs. Clin Endocrinol 1979;11:585-94.
- Feldt-Rasmussen U, Petersen PH, Date J and Madsen CM. Serum thyroglobulin in
patients undergoing subtotal thyroidectomy for toxic and nontoxic goiter. J
Endocrinol Invest 1982;5:161-4.
- Hocevar M, Auersperg M and Stanovnik L. The dynamics of serum thyroglobulin
elimination from the body after thyroid surgery. 1997;23:208-10.
- Cohen JH, Ingbar SH and Braverman LE. Thyrotoxicosis due to ingestion of
excess thyroid hormone. Endocrine Rev 1989;10:113-24.
- Mitchell ML and Hermos RJ. Measurement of thyroglobulin in newborn screening
specimens from normal and hypothyroid infants. Clin Endocrinol 1995;42:523-7.
- Smallridge RC, De Keyser FM, Van Herle AJ, Butkus NE and Wartofsky L.
Thyroid iodine content and serum thyroglobulin: clues to the natural history of
destruction-induced thyroiditis. J Clin Endocrinol Metab 1986;62:1213-9.
- Pacini F, Molinaro E, Lippi F, Castagna MG, Agate L, Ceccarelli C, Taddei D,
Elisei R, Capezzone M and Pinchera A. Prediction of disease status by
recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of
differentiated thyroid carcinoma. J Clin Endocrinol Metab 2001;86:5686-90.
- Cobin RH. 1992. Medullary carcinoma of the thyroid. In Malignant tumors of
the thyroid: clinical concepts and controversies. S. D. Cobin RH, editor.
Springer-Verlag,, New York. 112-41.
- Dunn JT. When is a thyroid nodule a sproadic medullary carcinoma? J Clin
Endocrinol Metab1994;78:824-5.
- Pacini F, Fontanelli M, Fugazzola L and et. al. Routine measurement of serum
calcitonin in nodular thyroid diseases allows the preoperative diagnosis of
unsuspeted sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab
1994;78:826-9.
- Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E et al.
Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia
type 2A. Nature 1993;363:458-60.
- Hofstra RM, Landvaster RM, Ceccherini I et al. A mutation in the RET
proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic
medullary thyroid carcinoma. Nature1994;367:375-6.
- Heyningen van V. One gene-four syndromes. Nature 1994;367:319-20.
- Becker KL, Nylen ES, Cohen R and Snider RH. Calcitonin: structure, molecular
biology and actions. In: J.P. Beleziakian, L.E. Raisz, G.A.Rodan eds. Principle
of bone biology, Academic Press, San Diego 1996;:471-4.
- Motte P, Vauzelle P, Gardet P, Ghillani P, Caillou B, Parmentier C et al.
Construction and clinical validation of a sensitive and specific assay for
mature calcitonin using monoclonal anti-peptide antibodies. Clin Chim Acta
1988;174:35-54.
- Zink A, Blind E and Raue F. Determination of serum calcitonin by
immunometric two-site assays in normal subjects and patients with medullary
thyroid carcinoma. Eur J Clin Chem Biochem1992;30:831-5.
- Engelbach M, Gorges R, Forst T, Pfutzner A, Dawood R, Heerdt S, Kunt T,
Bockisch A and Beyer J. Improved diagnostic methods in the follow-up of
medullary thyroid carcinoma by highly specific calcitonin measurements. J Clin
Endocrinol Metab 2000;85:1890-4.
- Milhaud G, Tubiana M, Parmentier C and Coutris G. Epithelioma de la thyroide
secretant de la thyrocalcitonine. C.R. Acad. Sci (serie D), Paris
1968;266:608-10.
- Guilloteau D, Perdrisot D, Calmettes C and et. al. Diagnosis of medullary
carcinoma of the thyroid by calcitonin assay using monoclonal antibodies. J Clin
Endocrinol Metab 1990;71:1064-7.
- Niccoli P, Wion-Barbot N, Caron P and et.al. Interest of routine measurement
of serum calcitonin(CT): study in a large series of thyroidectomized patients. J
Clin Endocrinol Metab 1997;82:338-41.
- Wells SA, Baylin SB, Linehan W, Farrell RE, Cox EB, Cooper CW. Provocative
agents and the diagnosis of medullary carcinoma of the thyroid gland. Ann Surg
1978;188:139-41.
- Gagel RF. The abnormal pentagastrin test. Clin Endocrinol 1996;44:221-2.
- Wion-Barbot N, Schuffenecker I, Niccoli P et al. Results of the calcitonin
stimulation test in normal volunteers compared with genetically unaffected
members of MEN 2A and familial medullary thyroid carcinoma families. Ann
Endocrinol 1997;58:302-8.
- Barbot N, Calmettes C, Schuffenecker I et al. Pentagastrin stimulation test
and early diagnosis of medullary carcinoma using an immunoradiometric assay of
calcitonin: comparison with genetic screening in hereditary medullary thyroid
carcinoma. J Clin Endocrinol Metab 1994;78:114-20.
- Erdogan MF, Gullu S, Baskal N, Uysal AR, Kamel N, Erdogan G. Omeprazole:
calcitonin stimulation test for the diagnosis follow-up and family screening in
medullary carcinoma of the thyroid gland. Ann Surg 1997;188:139-41.
- Vieira AEF, Mello MP, Elias LLK et al. Molecular and biochemical screening
for the diagnosis and management of medullary thyroid carcinoma in multiple
endocrine neoplasia Type 2A. Horm Metab Res 2002;34:202-6.
- Wells SA, Chi DD, toshima K, Dehner LP, Coffin cm, Dowton SB, Ivanovich JL,
DeBenedetti MK,Dilley WG and Moley JF. Predictive DNA testing and prophylactic
thyroidectomy in patients at risk for multiple endocrine neoplasia type 2A. Ann
Surg 1994;220:237-50.
- Telander RL and Moir CR. Medullary thyroid carcinoma in children. Semin
Pediatr Surg 1994;3:188-93.
- Niccoli-Sire P, Murat A, Baudin E, Henry JF, Proye C, Bigorgne JC et al.
Early or prophylactic thyroidectomy in MEN2/FMTC gene carriers: results in 71
thyroidectomized patients. Eur J Endocrinol 1999;141:468-74.
- Niccoli-Sire P, Murat A, Rohmer V, Franc S, Chabrier G, Baldet L, Maes B,
Savagner F, Giraud S,Bezieau S, Kottler ML, Morange S and Conte-Devolx B.
Familial medullary thyroid carcinoma(FMTC) with non-cysteine RET mutations:
phenotype-genotype relationship in large series of patients. J Clin Endocrinol
Metab 2001;86:3756-53.
- Body JJ, Chanoine JP, Dumon JC and Delange F. Circulating calcitonin levels
in healthy children and subjects with congenital hypothyroidism from birth to
adolescence. J Clin Endocrinol Metab1993;77:565-7.
- Gharib H, Kao PC and Heath H. Determination of silica-purified plasma
calcitonin for the detection and management of medullary thyroid carcinoma:
comparison of two provocative tests. Mayo Clin Proc 1987;62:373-8.
- Telander R, Zimmerman D, Sizemore GW, van Heerden JA and Grant CS. Medullary
carcinoma in children. Results of early detection and surgery. Arch Surg
1989;124:841-3.
- Calmettes C, Ponder BA, Fisher JA and Raue F. Early diagnosis of multiple
endocrine neoplasia type 2syndrome: consensus statement. European community
concerted action: medullary thyroid carcinoma. Eur J Clin Invest 1992;22:755-60.
- Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A et al.
Prognostic factors for survival and biochemical cure in medullary thyroid
carcinoma: results in 899 patients. Clin Endocrinol1998;48:265-73.
- Machens A, Gimm O, Ukkat J et al. Improved prediction of calcitonin
normalization in medullary thyroid carcinoma patients by quantitative lymph node
analysis. Cancer 2000;88:1909-15.
- Fugazzola L, Pinchera A, Lucchetti F et al. Disappearence rate of serum
calcitonin after total thyroidectomy for medullary thyroid carcinoma. Internat J
Biolog Markers 1994;9:21-4.
- Vierhapper H, Raber W, Bieglmayer C and et.al. Routine measurement of plasma
calcitonin in nodular thyroid diseases. J Clin Endocrinol Metab 1997;82:1589-93.
- Fereira-Valbuena H, Fernandez de Arguello E, Campos G, Ryder E and
Avellaneda A. Serum concentration of calcium and calcitonin in hyperthyroidism
caused by Graves' disease. Invest Clin1991;32:109-14.
- Lips CJM, Hoppener JWM and Thijssen JHH. Medullary thyroid carcinoma: role
of genetic testing and calcitonin measurement. Ann Clin Biochem 2001;38:168-79.
- Niccoli P, Brunet Ph, Roubicek C, Roux F, Baudin E, Lejeune PJ et al.
Abnormal calcitonin basal levels and pentagastrin response in patients with
chronic renal failure on maintenance hemodialysis. Eur J Endocrinol
1995;132:75-81.
- Snider RH, Nylen ES and Becker KL. Procalcitonin and its component peptides
in systemic inflammation: immunochemical characterization. J Invest Med
1997;47:552-60.
- Russwurn S, Wiederhold M, Oberhoffer M et al. Molecular aspects and natural
source of Procalcitonin. Clin Chem Lab Med 1999;37:789-97.
- Niccoli P, Conte-Devolx B, Lejeune PJ, Carayon P, Henry JF, Roux F et al.
Hypercalcitoninemia in conditions other than medullary cancers of the thyroid.
Ann Endocrinol 1996;57:15-21.
- Baudin E, Bidart JM, Rougier P et al. Screening for multiple endocrine
neoplasia type 1 and hormonal production in apparently sporadic neuroendocrine
tumors. J Clin Endocrinol Metab 1999;84:114-20.
- DeLellis RA. C-Cell hyperplasia. In: Rosai J., Carangiu M.L., DeLellis R.A.
eds: Atlas of Tumor Pathology, 3rd. series, Fasc 5: tumors of the thyroid gland.
Washington DC, Armed Forces Institute of Pathology. 1992;:247-58.
- Guyetant S, Wion-Barbot N and Rousselet MC. C-cell hyperplasia associated
with chronic lymphocytic thyroiditis: a retrospective study of 112 cases. Hum
Pathol 1994;25:514-21.
- Albores-Saavedra J, Monforte H, Nadji M and Morales AR. C-Cell hyperplasia
in thyroid tissue adjacent to follicular cell tumor. Hum Pathol 1988;19:795-9.
- Mulligan LM, Marsh DJ, Robinson BG, Schuffenecker I, Zedenius J, Lips CJ et
al. Genotype-phenotype correlation in multiple endocrine neoplasia type 2:
report of the international RET mutation consortium. J Intern Med
1995;238:243-6.
- Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF et al. The
relationship between specific RET proto-oncogene mutations and disease phenotype
in multiple endocrine neoplasia type 2. International RET mutation consortium
analysis. JAMA 1996;276:1575-9.
- Ito S, Iwashita T, Asai N, Murakami H, Iwata Y, Sobue G et al. Biological
properties of RET with cysteine mutations correlate with multiple endocrine
neoplasia type 2A, familial medullary thyroid carcinoma and Hirschsprung's
disease phenotype. Cancer Res 1997;57:2870-2.
- Heshmati HM, Gharib H, Khosla S et al. Genetic testing in medullary thyroid
carcinoma syndromes:mutation types and clinical significance. Mayo Clin Proc
1997;72:430-6.
- Berndt I, Reuter M, Saller B et al. A new hot spot for mutations in the RET
proto-oncogene causing familial medullary thyroid carcinoma and multiple
endocrine neoplasia type 2A. J Clin Endocrinol Metab 1998;83:770-4.
- Komminoth P, Roth J, Muletta-Feurer S, Saremaslani P, Seelentag WKF and
Heitz PU. RET proto-oncogene point mutations in sporadic neuroendocrine tumors.
J Clin Endocrinol Metab 1996;81:2041-6.
- Conte-Devolx B, Schuffenecker I, Niccoli P, Maes B, Boneu A, Barbot N et al.
Multiple Endocrine Neoplasia Type 2: Management of patients and subjects at
risk. Horm Res 1997;47:221-6.
- Smith DP, Houghton C and Ponder BA. Germline mutation of RET codon 883 in
two cases of de novo MEN2B. Oncogene 1997;15:1213-7.
- Carlson KM, Bracamontes J, Jackson CE, Clark R, Lacroix A, Wells SA Jr et
al. Parent-of-origin effects in multiple endocrine neoplasia type 2B. J Hum
Genet 1994;55:1076-82.
- Moers AMJ, Landsvater RM, Schaap C, van Veen JM, de Valk IAJ, Blijham GH et
al. Familial medullary thyroid carcinoma: not a distinct entity/
Genotype-phenotype correlation in a large family:familial medullary thyroid
carcinoma revisited. Am J Med 1996;101:634-41.
- Dunn JT. Iodine deficiency - the next target for elimination. N Engl J Med
1992;326:267-8.
- Delange F. Correction of iodine deficiency: benefits and possible side
effects. Eur J Endocrinol1995;132:542-3.
- Dunn JT. Whats happening to our iodine. J Clin Endocrinol Metab
1998;83:3398-3400.
- Knudsen N, Christiansen E, Brandt-Christensen M, Nygaard B and Perrild H.
Age- and sex-adjusted iodine/creatinine ratio. A new standard in epidemiological
surveys? Evaluation of three different estimates of iodine excretion based on
casual urine samples and comparison to 24 h values. Eur J Clin Nutr
2000;54:361-3.
- Aumont G and Tressol JC. Improved routine method for the determination of
total iodine in urine and milk. Analyst 1986;111:841-3.
- Unak P, Darcan S, Yurt F, Biber Z and Coker M. Determination of iodine
amounts in urine and water by isotope dilution analysis. Biol Trace Elem Res
Winter 1999;71-2:463-70.
- Kilbane MT, Ajja RA, Weetman AP, Dwyer R, McDermott EWM, O'Higfins NJ and
Smyth PPA. Tissue Iodine content and serum mediated 125I uptake blocking
activityin breast cancer. J Clin Endocrinol Metab 2000;85:1245-50.
- Liberman CS, Pino SC, Fang SL, Braverman LE and Emerson CH. Circulating
iodine concentrations during and after pregnancy. J Clin Endocrinol Metab
1998;83:3545-9.
- Vought RL, London WT, Lutwak L and Dublin TD. Reliability of estimates of
serum inorganic iodine and daily faecal and urinary iodine excretion from single
casual specimens. J Clin Endcorinol Metab1963;23:1218-28.
- Smyth PPA, Darke C, Parkes AB, Smith DF, Hetherton AM and Lazarus JH.
Assessment of goitre in an area of endemic iodine deficiency. Thyroid
1999;9:895-901.
- Thomson CD, Smith TE, Butler KA and Packer MA. An evaluation of urinary
measures of iodine and selenium status. J Trace Elem Med and Biol
1996;10:214-22.
- Als C, Helbling A, Peter K, Haldimann M, Zimmerli B and Gerber H. Urinary
iodine concentration follows a circadian rhythm: A study with 3023 spot urine
samples in adults and children. J Clin Endocrinol Metab 2000;85:1367-9.
- Lightowler H and Davis JG. Iodine intake and iodine deficiency in vegans as
assessed by the duplicate-portion technique and urinary iodine excretion. Br. J
Nutr 1999;80:529-35.
- Utiger RD. Maternal hypothyroidism and fetal development. N Engl J Med
1999;341:601-2.
- Aboul-Khair S, Crooks J, Turnbull AC and Hytten FE. The physiological
changes in thyroid function during pregnancy. Clin Sci 1964;27:195-207.
- Smyth PPA, Smith DF, Radcliff M and O'Herlihy C. Maternal iodine status and
thyroid volume during pregnancy: correlation with neonatal intake. J Clin
Endocrinol Metab 1997;82:2840-3.
- Gunton JE, Hams GH, Fiegert M and McElduff A. iodine deficiency in
ambulatory participants at a Sydney teaching hospital: Is Australia truly iodine
replete? Med J Aust 1999;171:467-70.
- Smyth PPA. Variation in iodine handling during normal pregnancy. Thyroid
1999;9:637-42.
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K,
Arsenic, Boron,Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel,
Silicon, Vanadium and Zinc. National Academic Press 2001
- Koutras DA, Papadoupoulos SN, Sfontouris JG and Rigopoulos GA. Comparison of
methods for measuring the plasma inorganic iodine and the absolute iodine uptake
by the thyroid gland. J Clin Endocrinol Metab 1968;28:757-60.
- Mizukami Y, Michigishi T, Nonomura A, Hashimoto T, Tonami N, Matsubara F et
al. Iodine-induced hypothyroidism: a clinical and histological study of 28
patients. J Clin Endocrinol Metab 1993;76:466-71.
- Heymann WR. Potassium iodide and the Wolff-Chaikhoff effect: relevance for
the dermatologist. J Am Acad Dermatol 2000;42:490-2.s
- Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Bidor G,
Braverman LE and Medeiros-Neto G. Iodine-induced hyperthyroidism: occurrence and
epidemiology. Thyroid 1998;8:83-100.
- Roti E and Uberti ED. Iodine excess and hyperthyroidism. Thyroid
2001;5:493-500.
- Baltisberger BL, Minder CE and Burgi H. Decrease of incidence of toxic
nodular goitre in a region of Switzerland after full correction of mild iodine
deficiency. Eur J Endocrinol 1995;132:546-9.
- Bacher-Stier RG, Totsch M, Kemmler G, Oberaigner W and Moncayo R. Incidence
and clinical characteristics of thyroid carcinoma after iodine prophylazis in an
endemic goiter country. Thyroid1997;7:733-41.
- Barakat MCD, Hetherton AM, Smyth PPA and Leslie H. Hypothyroidism secondary
to topical iodine treatment in infants with spina bifida. Acta Paediat
1994;83:741-3.
- Martino E, Safran M, Aghino-Lombardi F, Rajatanavin R, Lenziardi M, Fay M et
al. Environmental iodine intake and thyroid dysfunction during chronic
amiodarone therapy. Ann Intern Med1984;101:28-34.
- Rose NR, Rasooly L, Saboori AM and Burek CL. Linking iodine with autoimmune
thyroiditis. Environmental Health Perspectives 1999;107:749-52.
- Premawardhana LDKEPA, Smyth PPA, Wijeyaratne C, Jayasinghe A, De Silva H and
Lazarus JH. Increased prevalence of thyroglobulin antibodies in Sri Lankan
schoolgirls - is iodine the cause? Eur J Endocrinol 2000;143:185-8.
- Costa A, Testori OB, Cenderelli C, Giribone G and Migliardi M. Iodine
content of human tissues after administration of iodine containing drugs or
constrast media. J Endocrinol Invest 1978;1:221-5.
- May W, Wu D, Eastman C, Bourdoux P and Maberly G. Evaluation of automated
urinary iodine methods: problems of interfering substances identified. Clin Chem
1990;35:865-9.
- Lauber K. Iodine determination in biological material. Kinetic measurement
of the catalytic activity of iodine. Analyt Chem 1975;47:769-71.
- Mantel M. Improved method for the determination of iodine in urine. Clin
Chim Acta 1971;33:39-44.
- Dunn JT, Crutchfield HE, Gutenkunst R and Dunn AD. Two simple methods for
measuring iodine in urine. Thyroid 1993;3:119-23.
- May SL, May WA, Bourdoux PP, Pino S, Sullivan KM and Maberly GF. Validation
of a simple,manual urinary iodine method for estimating the prevalence of
iodine-deficiency disorders and interlaboratory comparison with other methods. J
Clin Nutr 1997;65:1441-5.
- Ohashi T, Yamaki M, Pandav SC, Karmarkar GM and Irie M. Simple microplate
method for determination of urinary iodine. Clin Chem 2000;46:529-36.
- Rendl J, Seybold S and Borner W. Urinary iodine determined by paired-ion
reverse-phase HPLC with electrochemical detection. Clin Chem 1994;40:908-13.
- Tsuda K, Namba H, Nomura T, Yokoyama N, Yamashita S, Izumi M and Nagataki S.
Automated Measurement of urinary iodine with use of ultraviolet radiation. Clin
Chem 1995;41:581-5.
- Haldimann M, Zimmerli B, Als C and Gerber H. Direct determination of urinary
iodine by inductively coupled plasma mass spectormetry using isotope dilution
with iodine-129. Clin Chem 1998;44:817-24.
- Mura P, Piriou A, Guillard O, Sudre Y and Reiss D. Dosage des iodures
urinares par electrode specifique: son interet au cours des dysthyroides. Ann
Biol Clin 1985;44:123-6.
- Allain P, Berre S, Krari N, Laine-Cessac P, Le Bouil A, Barbot N, Rohmer V
and Bigorgne JC. Use of plasma iodine assays for diagnosing thyroid disorders. J
Clin Pathol 1993;46:453-5.
- Vander JB, Gaston EA and Dawber TR. The significance of nontoxic thyroid
nodules: Final report of a15-year study of the incidence of thyroid malignancy.
Ann Intern Med 1968;69:537-40.
- Rojeski MT and Gharib H. Nodular thyroid disease: Evaluation and management.
N Engl J Med1985;313:428-36.
- Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med
1993;328:553-9.
- Kirkland RT and Kirkland JL. Solitary thyroid nodules in 30 children and
report of a child with thyroid abscess. Pediatrics 1973;51:85-90.
- Rallison ML, Dobyns EM, Keating FR, Rall J and Tyler E. Thyroid nodularity
in children. JAMA1975;233:1069-72.
- Khurana KK, Labrador E, Izquierdo R, Mesonero CE and Pisharodi LR. The role
of fine-needle aspiration biopsy in the management of thyroid nodules in
children, adolescents and young adults: A multi-institutional study. Thyroid
1999;4:383-6.
- Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, Leoli F,
Rago T, Grasso L,Valeriano R, Balestrieri A and Pinchera A. The spectrum of
thyroid disorders in an iodine-deficient community: the Pescopanano Survey. J
Clin Endocrinol Metab 1999;84:561-6.
- Hamburger JI, Husain M, Nishiyama R, Nunez C and Solomon D. Increasing the
accuracy of fine-needle biopsy for thyroid nodules. Arch Pathol Lab Med
1989;113:1035-41.
- Hundahl SA, Cady B, cunningham MP, Mazzaferri E, McKee RF, Rosai J, Shah JP,
Fremgen AM,Stewart AK and Holzer S. Initial results from a prospective cohort
study of 5583 cases of thyroid carcinoma treated in the United States during
1996. Cancer (Cytopathol) 2000;89:202-17.
- Leenhardt L, Hejblum G, Franc B, Du Pasqueir Fediaevsky L, Delbot T, De
Guillouzic D, Menegaux F, Guillausseau C, Hoang C, Turpin G and Aurengo A.
Indications and limits of ultrasound-guided cytology in the management of
nonpalpable thyroid nodules. J Clin Endocrinol Metab 1999;84:24-8.
- Braga M, Cavalcanti TC, Collaco LM and Graf H. Efficacy of ultrasound-guided
fine-needle aspiration biopsy in the diagnosis of complex thyroid nodules. J
Clin Endocrinol Metab 2001;86:4089-91.
- Cochand-Priollet B, Guillausseau P, Chagnon S, Hoang C, Guillausseau-Scholer
C, Chanson P, Dahan H, Warnet A, Tran Ba Huy PT and Valleur P. The diagnostic
value of fine-needle aspiration biopsy under ultrasonoraphy in nonfunctional
thyroid nodules: a prospective study comparing cytologic and histologic
findings. Am J Med 1994;97:152-7.
- Takashima S, Fukuda H and Kobayashi T. Thyroid nodules: Clinical effect of
ultrasound-guided fine needle aspiration biopsy. J Clin Ultrasound
1994;22:535-42.
- Gharib H. Fine-needle aspiration biopsy of thyroid nodules: Advantages,
limitations and effect. Mayo Clin Proc 1994;69:44-9.
- Hamberger B, Gharib H, Melton LF III, Goellner JR and zinsmeister AR.
Fine-needle aspiration biopsy of thyroid nodules. Impact on thyroid practice and
cost of care. Am J Med 1982;73:381-4.
- Grant CS, Hay ID, Gough IR, McCarthy PM and Goelliner JR. Long-term
follow-up of patients with benign thyroid fine-needle aspiration cytologic
diagnoses. Surgery 1989;106:980-6.
- Liel Y and Barchana M. Long-term follow-up of patients with initially benign
fine-needle aspirations. Thyroid 2001;11:775-8.
- Belfiore A, La Rosa G, La Porta GA, Giuffrida D, Milazzo G, Lupo L,
Regalbuto C and V. R. Cancer Risk in patients with cold thyroid nodules:
Relevance of iodine intake, sex, age and multinodularity. J Amer Med
1992;93:363-9.
- Tuttle RM, Lemar H and Burch HB. Clinical features associated with an
increased risk of thyroid malignancy in patients with follicular neoplasia by
fine-needle aspiration. Thyroid 1998;8:377-83.
- Kumar H, Daykin J, Holder R, Watkinson JC, Sheppard M and Franklyn JA.
Gender, clinical findings and serum thyrotropin measurements in the prediction
of thyroid neoplasia in 1005 patients presenting with thyroid enlargement and
investigated by fine-needle aspiration cytology. Thyroid 1999;11:1105-9.
- Moosa M and Mazzaferri EL. Outcome of differentiated thyroid cancer
diagnosed in pregnant women. J Clin Endocrinol Metab 1997;82:2862-6.
- Oertel YC. A pathologist trying to help endocrinologists to interpret
cytology reports from thyroid aspirates. J Clin Endocrinol Metab
2002;87:1459-61.
- De Micco, Zoro P, Garcia S, Skoog L, Tani EM, C. PK and Henry JF. Thyroid
peroxidase immunodetection as a tool to assist diagnosis of thyroid nodules on
fine-needle aspiration biopsy. Eur J Endocrinol 1994;131:474-9.
- Faroux MJ, Theobald S, Pluot M, Patey M and Menzies D. Evaluation of the
monoclonal antithyroperoxidase MoAb47 in the diagnostic decision of cold thyroid
nodules by fine-needle aspiration. Pathol Res Pract 1997;193:705-12.
- Inohara H, Honjo Y, Yoshii T, Akahani S, Yoshida J, Hattori K, Okamoto S,
Sawada T, Raz A and Kubo T. Expression of galectin-3 in fine-needle aspirates as
a diagnostic marker differentiating benign from malignant thyroid neoplasms.
Cancer 1999;85:2475-84.
- Medeiros-Neto G, Nascimento MC, Bisi H, Alves VA, Longatto-Filho A and
Kanamura CT. Differential reactivity for Galectin-3 in Hurthle Cell Adenomas and
Carcinomas. Endocr Pathol2001;12:275-9.
- Saggiorato E, Cappia S, De Guili P, Mussa A, Pancani G, Caraci P, Angeli A
and Orlandi F. Galectin -3 as a presurgical immunocytodiagnostic marker of
minimally invasive follicular carcinoma. J Clin Endocrinol Metabl
2001;86:5152-8.
- Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A, Inohara
H, Marandino F,Orkandi F, Nardi F, Vacchione A, Tecce R and Larsson O.
Application of an immunodiagnostic method for improving preoperative diagnosis
of nodular thyroid lesions. Lancet 2001;357:1644-50.
- Goellner JR. Problems and pitfalls in thyroid cytology. Monogr Pathol
1997;39:75-93.
- Oertel YC, O. J. Diagnosis of benign thyroid lesions: fine-needle aspiration
and histopathologic correlation. Ann Diagn Pathol 1998;2:250-63.
- Baldet L, Manderscheid JC, Glinoer D, Jaffiol C, Coste-Seignovert B and
Percheron C. The management of differentiated thyroid cancer in Europe in 1988.
Results of an international survey. Acta Endocrinol (Copenh) 1989;120:547-58.
- Baloch ZW, Fleisher S, LiVolsi VA and Gupta PK. Diagnosis of "follicular
neoplasm": a gray zone in thyroid fine-needle aspiration cytology. Diagn
Cytopathol 2002;26:41-4.
- Herrmann ME, LiVolsi VA, Pasha TL, Roberts SA, Wojcik EM and Baloch ZW.
Immunohistochemical expression of Galectin-3 in benign and malignant thyroid
lesions. Arch Pathol Lab Med 2002;126:710-13.
- Leteurtre E, Leroy Z, Pattou F, Wacrenier A, Carnaille B, Proye C and
Lecomte-Houcke M. Why do frozen sections have limited value in encapsulated or
minimally invasive follicular carcinoma of the thyroid? Amer J Clin Path
2001;115:370-4.
- Stojadinovic A, Ghossein RA, Hoos A, Urist MJ, Spiro RH, Shah JP, Brennan
MF, Shaha AR and Singh B. Hurthle cell carcinoma: a critical histopathologic
appraisal. J Clin Oncol 2001;19:2616-25.
- Carmeci C, Jeffrey RB, McDougall IR, Nowels KW and Weigel RJ.
Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid
1998;8:283-9.
- Yang GCH, Liebeskind D and Messina AV. Ultrasound-guided fine-needle
aspiration of the thyroid assessed by ultrafast papanicoulaou stain: Data from
1135 biopsies with a two- six-year follow-up. Thyroid 2001;6:581-9.
- Fisher DA, Dussault JH, Foley TP, Klein AH, LaFranchi S, Larsen PR, Mitchell
NL, Murphey WH and Walfish PG. Screening for congenital hypothyroidism: results
of screening one million North American infants. J Pediatr 1979;94:700.
- Brown AL, Fernhoff PM, Milner J, McEwen C and Elsas LS. Racial differences
in the incidence of congenital hypothyroidism. J Pediatr 1981;99:934-.
- LaFranchi SH, Dussault JH, Fisher DA, Foley TP and Mitchell ML. Newborn
screening for congenital hypothyroidism: Recommended guidelines. Pediatrics
1993;91:1203-9.
- Gruters A, Delange F, Giovanelli G, Klett M, Richiccioli P, Torresani T et
al. Guidelines for neonatal screening programmes for congenital hypothyroidism.
Pediatr 1993;152:974-5.
- Toublanc JE. Guidelines for neonatal screening programs for congenital
hypothyroidism. Acta Paediatr1999;88 Suppl 432:13-4.
- Vulsma T, Gons MH and de Vijlder JJ. Maternal-fetal transfer of thyroxine in
congenital hypothyroidism due to a total organification defect or thyroid
agenesis. N Engl J Med 1989;321:13-6.
- Gruneiro-Papendieck L, Prieto L, Chiesa A, Bengolea S, Bossi G and Bergada
C. Usefulness of thyroxine and free thyroxine filter paper measurements in
neonatal screening for congenital hypothyroidism of preterm babies. J Med Screen
2000;7:78-81.
- Hanna DE, Krainz PL, Skeels MR, Miyahira RS, Sesser DE and LaFranchi SH.
Detection of congenital hypopituitary hypothyroidism: Ten year experience in the
Northwest Regional Screening Program. J Pediatr 1986;109:959-64.
- Fisher DA. Hypothyroxinemia in premature infants: is thyroxine treatment
necessary? Thyroid1999;9:715-20.
- Wang ST, Pizzalato S and Demshar HP. Diagnostic effectiveness of TSH
screening and of T4 with secondary TSH screening for newborn congenital
hypothyroidism. Clin Chim Acta 1998;274:151-8.
- Delange F. Screening for congenital hypothyroidism used as an indicator of
the degree of IDD and its control. Thyroid 1998;8:1185-92.
- Law WY, Bradley DM, Lazarus JH, John R and Gregory JW. Congenital
hypothyroidism in Wales(1982-93): demographic features, clinical presentation
and effects on early neurodevelopment. Clin Endocrinol 1998;48:201-7.
- Mei JV, Alexander JR, Adam BW and Hannon WH. Use of filter paper for the
collection and analysis of human whole blood specimens. J Nutr
2001;131:1631S-6S.
- LaFranchi SH, Hanna CE, Krainz PL, Skeels MR, Miyahira RS and Sesser DE.
Screening for congenital hypothyroidism with specimen collection at two time
periods: Results of the Northwest Regional Screening Program. J Pediatr
1985;76:734-40.
- Zakarija M, McKenzie JM and Eidson MS. Transient neonatal hypothyroidism:
Characterization of maternal antibodies to the Thyrotropin Receptor. J Clin
Endocrinol Metab 1990;70:1239-46.
- Matsuura N, Yamada Y, Nohara Y, Konishi J, Kasagi K, Endo K, Kojima H and
Wataya K. Familial neonatal transient hypothyroidism due to maternal TSH-binding
inhibitor immunoglobulins. N Engl J Med 1980;303:738-41.
- McKenzie JM and Zakaria M. Fetal and neonatal hyperthyroidism and
hypothyroidism due to maternal TSH receptor antibodies. Thyroid 1992;2:155-9.
- Vogiatzi MG and Kirkland JL. Frequency and necessity of thyroid function
tests in neonates and infants with congenital hypothyroidism. Pediatr 1997;100.
- Pohlenz J, Rosenthal IM, Weiss RE, Jhiang SM, Burant C and Refetoff S.
Congenital hypothyroidism due to mutations in the sodium/iodide symporter.
Identification of a nonsense mutation producing a downstream cryptic 3' splice
site. J Clin Invest 1998;101:1028-35.
- Nordyke RA, Reppun TS, Mandanay LD, Wood JC, Goldstein AP and Miyamoto LA.
Alternative sequences of thyrotropin and free thyroxine assays for routine
thyroid function testing. Quality and cost. Arch Intern Med
1998;158:266-72.
Page forms part of www.apls.tk, the information site on ANTIPHOSPHOLIPID SYNDROME (APS or ANTIPHOSPHOLIPID SYNDROME (APLS))
Medical Keywords: systemic antiphospholipid antibody syndrome, Antiphospholipid, Antiphospholipid Antibody Syndrome, Antiphospholipid Syndrome, APS, APLS, Hughes
Syndrome, Sticky Blood, Clotting Disorder, Stroke, TIA, PE, death, Antiphospholipid Antibody Syndrome, Antiphospholipid Syndrome, APS, APLS,
Hughes Syndrome, Sticky Blood, Clotting Disorder, Stroke, TIA, PE, death
|
|
|

